How Many Valence Electrons Are In Oxygen
Juapaving
Mar 28, 2025 · 5 min read
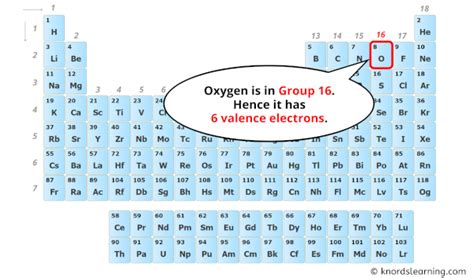
Table of Contents
How Many Valence Electrons Are in Oxygen? A Deep Dive into Atomic Structure and Bonding
Understanding the number of valence electrons in an atom is crucial for comprehending its chemical behavior and how it interacts with other atoms to form molecules and compounds. This article delves into the specifics of oxygen, exploring its atomic structure, electron configuration, and the significance of its valence electrons in forming chemical bonds. We’ll also touch upon related concepts like oxidation states and the role of oxygen in various chemical reactions.
Oxygen: A Cornerstone of Life and Chemistry
Oxygen (O), with its atomic number 8, is a highly reactive nonmetal and a vital component of the Earth's atmosphere and essential for most life forms. Its remarkable reactivity stems directly from its valence electron configuration, which dictates how it forms chemical bonds. Before we explore its valence electrons, let's briefly review some fundamental concepts.
Atomic Structure: Protons, Neutrons, and Electrons
Every atom consists of a nucleus containing protons (positively charged) and neutrons (neutral), surrounded by a cloud of orbiting electrons (negatively charged). The atomic number (Z) represents the number of protons in the nucleus and uniquely identifies an element. Oxygen's atomic number is 8, meaning it has 8 protons. In a neutral atom, the number of electrons equals the number of protons. Therefore, a neutral oxygen atom has 8 electrons.
Electron Shells and Subshells: Understanding Electron Configuration
Electrons are not randomly distributed around the nucleus but occupy specific energy levels or shells. These shells are further divided into subshells (s, p, d, and f), each capable of holding a specific number of electrons. The electron configuration describes how electrons are distributed among these shells and subshells.
Oxygen's electron configuration is 1s²2s²2p⁴. Let's break this down:
- 1s²: The first shell (n=1) contains one subshell, the 's' subshell, which holds a maximum of 2 electrons. Oxygen has 2 electrons in this innermost shell.
- 2s²: The second shell (n=2) also has an 's' subshell, holding another 2 electrons.
- 2p⁴: The second shell also contains a 'p' subshell, which can hold a maximum of 6 electrons. Oxygen has 4 electrons in its 2p subshell.
This configuration demonstrates the fundamental principle that electrons fill lower energy levels before occupying higher energy levels.
Valence Electrons: The Key to Chemical Bonding
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely held and are directly involved in chemical bonding. They determine an atom's reactivity and the types of bonds it can form (ionic, covalent, or metallic).
In oxygen's case, the outermost shell is the second shell (n=2), which contains both the 2s and 2p electrons. Therefore, oxygen has a total of six valence electrons (2 from the 2s subshell and 4 from the 2p subshell). This is a crucial piece of information for understanding its chemical behavior.
The Octet Rule and Oxygen's Reactivity
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell, similar to the noble gases. Oxygen, with its six valence electrons, needs to gain two more electrons to achieve a stable octet. This explains its high reactivity and tendency to form chemical bonds.
How Oxygen Achieves a Stable Octet: Bond Formation
Oxygen's strong tendency to gain two electrons leads to the formation of various chemical bonds:
Ionic Bonds: Electron Transfer
Oxygen can form ionic bonds with highly electropositive metals like sodium (Na) or magnesium (Mg). In these bonds, oxygen readily accepts two electrons from the metal, achieving a stable octet and forming a negatively charged ion called an oxide ion (O²⁻). The metal loses electrons, becoming a positively charged ion. The electrostatic attraction between these oppositely charged ions constitutes the ionic bond. An example is the formation of magnesium oxide (MgO): Mg²⁺ + O²⁻ → MgO
Covalent Bonds: Electron Sharing
Oxygen more frequently forms covalent bonds, sharing electrons with other nonmetals to achieve a stable octet. In a covalent bond, atoms share one or more pairs of electrons to fulfill the octet rule. A classic example is the formation of oxygen gas (O₂), where two oxygen atoms share two pairs of electrons to form a double bond, each atom effectively having eight electrons around it. This is denoted by the Lewis structure: O=O. Water (H₂O) is another prime example, where each oxygen atom forms two single covalent bonds with two hydrogen atoms.
Oxidation States and Oxygen's Role in Redox Reactions
The oxidation state (or oxidation number) is a measure of the apparent charge on an atom in a molecule or ion. It reflects the number of electrons an atom has gained or lost relative to its neutral state. In most of its compounds, oxygen exhibits an oxidation state of -2, reflecting its tendency to gain two electrons. However, there are exceptions, such as in peroxides (like hydrogen peroxide, H₂O₂), where oxygen has an oxidation state of -1.
Oxygen's high electronegativity (its ability to attract electrons in a bond) makes it a strong oxidizing agent. In redox reactions (reduction-oxidation reactions), oxygen readily accepts electrons (gets reduced), causing the other reactant to lose electrons (gets oxidized). This is fundamental to many processes, including combustion, respiration, and corrosion.
Oxygen's Importance in Various Fields
Oxygen's significance extends far beyond its role in basic chemistry. Its properties and reactivity are exploited in countless applications across various fields:
- Medicine: Oxygen therapy is crucial for patients with respiratory problems.
- Industry: Oxygen is used in welding, cutting, and other high-temperature processes.
- Environmental Science: Understanding oxygen's role in the environment is critical for studying climate change and pollution.
- Biology: Oxygen is essential for respiration in most living organisms.
Conclusion: The Significance of Oxygen's Valence Electrons
The presence of six valence electrons in oxygen profoundly impacts its chemistry. This electron configuration dictates its high reactivity, its tendency to form ionic or covalent bonds, and its role as a strong oxidizing agent. Understanding this fundamental aspect of oxygen's atomic structure is crucial for appreciating its widespread importance in various fields, from medicine and industry to the environment and life itself. The pursuit of knowledge in this area continues to drive advancements in scientific understanding and technological innovation. Further research into oxygen's behavior under extreme conditions and its interactions with other elements promises to reveal more about this ubiquitous and essential element.
Latest Posts
Latest Posts
-
How Far Is 20 Kilometers In Miles
Mar 31, 2025
-
How To Find The Perimeter Of Semicircle
Mar 31, 2025
-
What Is The Least Common Multiple Of 11 And 2
Mar 31, 2025
-
How Do You Calculate The Percent Abundance Of An Isotope
Mar 31, 2025
-
Equation Of A Circle Calculator Given Two Points
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
