What Are The Rows Of A Periodic Table Called
Juapaving
Mar 04, 2025 · 7 min read
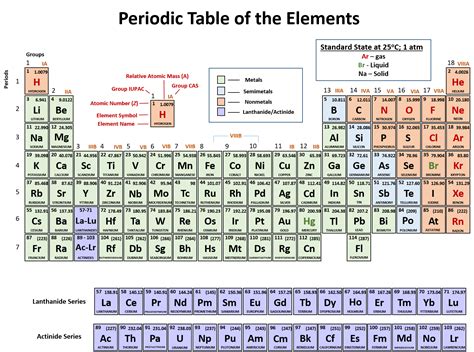
Table of Contents
What Are the Rows of a Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, that iconic chart adorning countless classrooms and laboratories, is more than just a colorful arrangement of elements. It's a meticulously organized system reflecting the fundamental properties and behaviors of matter. Understanding its structure is crucial to grasping the intricacies of chemistry. One of the most basic, yet essential, aspects of its structure is the arrangement of elements into rows. But what are these rows called? They're called periods. This article will delve deep into the concept of periods in the periodic table, exploring their significance, trends, and the underlying principles governing their arrangement.
Understanding Periods: A Horizontal Journey Through Atomic Structure
The periodic table is arranged in a grid, with elements organized into both horizontal rows and vertical columns. The horizontal rows are called periods, while the vertical columns are known as groups or families. Each period represents a principal energy level or shell in an atom. As we move across a period from left to right, the atomic number increases, meaning the number of protons and electrons in the atom increases sequentially.
Period 1: The Simplest Beginnings
Period 1, the shortest period, contains only two elements: hydrogen (H) and helium (He). These elements have their electrons filling the first principal energy level (n=1), which can only accommodate a maximum of two electrons. This small size and simple electronic structure contribute to their unique chemical properties. Hydrogen, being highly reactive, readily forms covalent bonds, while helium, with its full electron shell, is exceptionally inert, a noble gas.
Period 2: The Emergence of s and p Blocks
Period 2 is significantly longer, spanning from lithium (Li) to neon (Ne). This period introduces the s and p atomic orbitals. The alkali metal lithium begins the period, initiating the filling of the 2s orbital. As we traverse the period, subsequent elements fill the 2p orbitals, leading to a greater diversity of electronic configurations and chemical behaviors. The period concludes with the noble gas neon, exhibiting exceptional stability due to a complete octet of electrons in its valence shell (2s²2p⁶).
Period 3: Expanding the p Block
Similar to Period 2, Period 3 begins with an alkali metal, sodium (Na), and ends with a noble gas, argon (Ar). However, this period further expands the p block, accommodating more electrons. The increased number of electrons impacts the size and shielding effects, subtly altering the chemical properties compared to Period 2 elements. This period illustrates the trends in properties like electronegativity, ionization energy, and atomic radius as we proceed from left to right.
Periods 4-7: The Introduction of d and f Blocks
Periods 4 through 7 mark a significant shift in complexity. These periods witness the introduction of d and f orbitals, resulting in much longer periods. These orbitals are characterized by their lower energy levels and increased shielding effects. The d block elements, known as transition metals, exhibit variable oxidation states and form colorful complex ions. The f block elements, the lanthanides and actinides, are situated separately at the bottom of the periodic table due to their similar chemical properties and to maintain the overall table structure.
Trends Across Periods: A Systematic Variation
Moving across a period reveals fascinating trends in the properties of elements. These trends are directly linked to the increasing nuclear charge and the subtle changes in the electron configurations.
Atomic Radius: A Steady Decrease
The atomic radius generally decreases across a period. As the number of protons increases, the nuclear charge increases, pulling the electrons closer to the nucleus, thereby reducing the atomic size. This trend is evident from the alkali metal at the beginning of each period to the halogens towards the end.
Ionization Energy: An Upward Climb
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. This increase is attributed to the increased nuclear charge, making it more difficult to remove an electron from the increasingly positively charged nucleus. Exceptions can occur due to electron-electron repulsions in specific orbitals.
Electronegativity: A Similar Ascent
Electronegativity, the ability of an atom to attract electrons in a chemical bond, also generally increases across a period. The increased nuclear charge enhances the atom's pull on bonding electrons, making it more electronegative. This trend significantly influences the nature of chemical bonds formed by elements within a period.
Metallic Character: A Gradual Decline
Metallic character, typically associated with properties like conductivity and malleability, generally decreases across a period. As we move from left to right, the elements become less metallic and more non-metallic in their characteristics, reflecting the increasing tendency to gain electrons rather than lose them.
The Significance of Periods in Understanding Chemical Reactivity
The periodic arrangement into periods is not just an aesthetic choice. It's fundamental to understanding the chemical reactivity of elements. The number of valence electrons, the electrons in the outermost shell, largely determines an element's reactivity. Elements within the same period have valence electrons in the same principal energy level, but with varying numbers. This affects their bonding behavior and the types of compounds they form.
For instance, alkali metals in the first column of each period (Group 1) have only one valence electron, making them highly reactive as they readily lose this electron to achieve a stable electron configuration. Conversely, halogens in the seventh column (Group 17) have seven valence electrons, making them highly reactive as they tend to gain one electron to complete their octet. The noble gases in the eighth column (Group 18), with their full valence shells, exhibit very low reactivity, hence their designation as “noble gases.”
Periods and the Prediction of Chemical Properties
The periodic table, with its arrangement into periods, enables us to predict the chemical properties of elements based on their position. Knowing the period an element belongs to provides insights into its atomic structure, valence electron configuration, and the types of chemical bonds it is likely to form. This predictive power is invaluable in chemistry, aiding in the understanding of chemical reactions and the synthesis of new materials.
Beyond the Basics: Advanced Concepts Related to Periods
The organization of elements into periods forms the foundation for understanding more advanced concepts in chemistry. These concepts build upon the basic principles of periods and further refine our understanding of matter.
Periodicity: The Recurring Patterns of Properties
The repeating patterns of properties observed across periods are collectively known as periodicity. These patterns are the direct consequence of the repeating filling of electron shells, leading to similar valence electron configurations and related chemical behaviors in elements of the same group (column).
Valence Shell Electron Pair Repulsion (VSEPR) Theory
VSEPR theory uses the arrangement of valence electrons to predict the three-dimensional shapes of molecules. The number of valence electrons, determined by the element's position within a period, plays a crucial role in determining the molecular geometry.
Quantum Mechanics and Electron Configuration
The quantum mechanical model explains the arrangement of electrons within atoms, including their distribution in different energy levels and orbitals. This model forms the theoretical foundation for the organization of elements into periods, as each period corresponds to the filling of a particular principal energy level.
Conclusion: Periods – The Cornerstone of the Periodic Table
The rows of the periodic table, known as periods, represent a fundamental aspect of its organization and are vital to understanding the behavior of chemical elements. The systematic variation in atomic structure across a period directly impacts the physical and chemical properties of elements, leading to the predictable trends observed in atomic radius, ionization energy, electronegativity, and metallic character. Understanding periods is crucial for predicting the reactivity of elements, understanding chemical bonding, and interpreting more advanced chemical concepts. By appreciating the significance of periods, we gain a deeper understanding of the periodic table's power as a tool for organizing and interpreting the vast world of chemical elements. The periodic table's structure, especially the concept of periods, isn't merely a descriptive tool; it's a powerful predictive model that continues to shape our understanding of the fundamental building blocks of matter.
Latest Posts
Latest Posts
-
Which Is Greater Megabytes Or Gigabytes
Mar 04, 2025
-
What Is The Lcm For 10 And 12
Mar 04, 2025
-
Which Element Has Highest Ionization Energy
Mar 04, 2025
-
Least Common Factor Of 3 And 5
Mar 04, 2025
-
The Structural And Functional Units Of All Living Organisms
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about What Are The Rows Of A Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
