What Are The Four Major Classes Of Biomolecules
Juapaving
Mar 29, 2025 · 7 min read
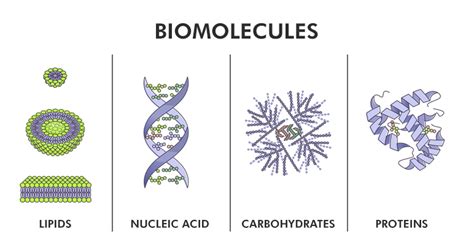
Table of Contents
What Are the Four Major Classes of Biomolecules?
Life, in all its stunning complexity, boils down to the intricate interplay of four major classes of biomolecules: carbohydrates, lipids, proteins, and nucleic acids. These molecules are the fundamental building blocks of all living organisms, each playing a crucial role in structure, function, and regulation. Understanding their individual properties and collective interactions is key to understanding the very essence of life itself. This article will delve deep into each class, exploring their structure, function, and importance in biological systems.
1. Carbohydrates: The Energy Source and Structural Scaffold
Carbohydrates, often referred to as saccharides, are the most abundant organic molecules on Earth. Their primary function is to provide energy, but they also play critical structural roles in various organisms. Carbohydrates are composed of carbon, hydrogen, and oxygen atoms, usually in a ratio of 1:2:1 (hence the name, "hydrates of carbon"). They are classified based on their size and structure:
1.1 Monosaccharides: The Simple Sugars
Monosaccharides are the simplest form of carbohydrates, acting as the building blocks for larger carbohydrate structures. They are typically characterized by a single sugar unit and cannot be further hydrolyzed into smaller sugars. Common examples include:
- Glucose: The primary energy source for cells, glucose is crucial for cellular respiration and ATP production. It's found in fruits, honey, and is a component of starch and cellulose.
- Fructose: Found in fruits and honey, fructose is a sweeter monosaccharide than glucose. It plays a role in energy metabolism.
- Galactose: Less sweet than glucose or fructose, galactose is a component of lactose (milk sugar).
1.2 Disaccharides: Two Sugars Joined
Disaccharides are formed by the joining of two monosaccharides through a glycosidic bond, a process involving the removal of a water molecule (dehydration synthesis). Examples include:
- Sucrose: Table sugar, composed of glucose and fructose.
- Lactose: Milk sugar, composed of glucose and galactose.
- Maltose: Malt sugar, composed of two glucose units.
1.3 Polysaccharides: Complex Carbohydrates
Polysaccharides are long chains of monosaccharides linked together by glycosidic bonds. They serve diverse functions, including energy storage and structural support. Important examples are:
- Starch: The primary energy storage polysaccharide in plants, consisting of amylose (a linear chain) and amylopectin (a branched chain) of glucose units.
- Glycogen: The primary energy storage polysaccharide in animals, stored mainly in the liver and muscles. It's a highly branched structure of glucose units.
- Cellulose: A major structural component of plant cell walls. It's a linear chain of glucose units linked by β-1,4-glycosidic bonds, which makes it indigestible by humans due to the lack of the necessary enzymes to break these bonds.
- Chitin: A structural polysaccharide found in the exoskeletons of arthropods and the cell walls of fungi. It's similar to cellulose but contains a nitrogen-containing group.
2. Lipids: Diverse Roles in Structure and Function
Lipids are a diverse group of hydrophobic (water-insoluble) biomolecules, characterized by their insolubility in water and solubility in nonpolar solvents. They play crucial roles in energy storage, membrane structure, hormone signaling, and insulation. Key lipid classes include:
2.1 Triglycerides: Energy Storage Champions
Triglycerides are the most common type of lipid, composed of a glycerol molecule esterified to three fatty acid chains. They serve as the primary energy storage molecules in animals and plants. Fatty acids can be saturated (no double bonds between carbon atoms), monounsaturated (one double bond), or polyunsaturated (multiple double bonds). The degree of saturation influences the lipid's physical properties, such as melting point.
2.2 Phospholipids: The Membrane Architects
Phospholipids are crucial components of cell membranes. They have a hydrophilic (water-loving) head and two hydrophobic (water-fearing) tails, forming a lipid bilayer that separates the cell's interior from its external environment. This structure allows for selective permeability, regulating the passage of molecules into and out of the cell.
2.3 Steroids: Signaling Molecules and Structural Components
Steroids are lipids characterized by a four-ring structure. Cholesterol is a crucial steroid, a component of cell membranes and a precursor for many steroid hormones, including testosterone, estrogen, and cortisol. These hormones play vital roles in regulating various physiological processes.
2.4 Waxes: Protective Coatings
Waxes are long-chain esters formed from fatty acids and long-chain alcohols. They provide a protective coating for plants and animals, preventing water loss and protecting against pathogens. Examples include beeswax and the waxy cuticle on plant leaves.
3. Proteins: The Workhorses of the Cell
Proteins are the most diverse class of biomolecules, playing a vast array of roles in virtually every biological process. They are polymers of amino acids, linked together by peptide bonds. The sequence of amino acids determines a protein's unique three-dimensional structure, which dictates its function. Proteins are classified based on their structure and function:
3.1 Amino Acids: The Building Blocks
Amino acids are the monomers of proteins. There are 20 different amino acids, each with a unique side chain (R-group) that contributes to its properties. These properties influence how the protein folds and functions.
3.2 Protein Structure: From Primary to Quaternary
Protein structure is hierarchical, with four levels of organization:
- Primary Structure: The linear sequence of amino acids.
- Secondary Structure: Local folding patterns, such as alpha-helices and beta-sheets, stabilized by hydrogen bonds.
- Tertiary Structure: The overall three-dimensional arrangement of a polypeptide chain, stabilized by various interactions between amino acid side chains (hydrophobic interactions, hydrogen bonds, disulfide bonds, ionic bonds).
- Quaternary Structure: The arrangement of multiple polypeptide chains (subunits) in a protein complex.
3.3 Protein Function: A Diverse Repertoire
Proteins perform a vast array of functions, including:
- Enzymes: Catalyze biochemical reactions.
- Structural Proteins: Provide support and structure (e.g., collagen, keratin).
- Transport Proteins: Carry molecules across cell membranes (e.g., hemoglobin).
- Hormones: Chemical messengers that regulate physiological processes.
- Receptor Proteins: Bind to signaling molecules and initiate cellular responses.
- Antibodies: Part of the immune system, protecting against pathogens.
- Motor Proteins: Involved in movement (e.g., myosin, kinesin).
4. Nucleic Acids: The Information Carriers
Nucleic acids, DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), are the information-carrying molecules of the cell. They store, transmit, and express genetic information, directing the synthesis of proteins and regulating cellular processes. Nucleic acids are polymers of nucleotides:
4.1 Nucleotides: The Building Blocks
Nucleotides are composed of a nitrogenous base (adenine, guanine, cytosine, thymine in DNA; uracil replaces thymine in RNA), a pentose sugar (deoxyribose in DNA, ribose in RNA), and a phosphate group.
4.2 DNA: The Blueprint of Life
DNA is a double-stranded helix, with the two strands held together by hydrogen bonds between complementary base pairs (A with T, and G with C). The sequence of bases in DNA encodes the genetic information, providing the blueprint for the synthesis of proteins and regulating gene expression.
4.3 RNA: The Messenger and Executor
RNA plays various roles in gene expression. Messenger RNA (mRNA) carries the genetic code from DNA to ribosomes, where it directs protein synthesis. Transfer RNA (tRNA) carries amino acids to the ribosomes, and ribosomal RNA (rRNA) is a structural component of ribosomes. Other types of RNA, such as microRNA (miRNA), play roles in gene regulation.
Interplay and Conclusion
These four classes of biomolecules are not isolated entities but rather work together in a highly coordinated manner. For instance, carbohydrates provide energy for protein synthesis, lipids form the membranes that compartmentalize cellular processes, proteins carry out the instructions encoded in nucleic acids, and nucleic acids direct the synthesis of proteins that are vital for all cellular functions. The intricate interactions and relationships between these biomolecules are essential for the maintenance of life and its remarkable diversity. Further research continually unveils new facets of their complexities and contributions to the overall biological landscape. Understanding the structure and function of these four biomolecule classes is foundational to grasping the intricacies of life itself, from the molecular level to the macroscopic world of organisms.
Latest Posts
Latest Posts
-
What 2 Subatomic Particles Make Up The Nucleus
Apr 01, 2025
-
How Many Inches Are 50 Cm
Apr 01, 2025
-
What Is The Lcm Of 4 And 5
Apr 01, 2025
-
What Is The Symbol For Magnesium
Apr 01, 2025
-
Moral Of The Lion And The Mouse Story
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Are The Four Major Classes Of Biomolecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
