What Are The Components Of A Nucleotide
Juapaving
Mar 10, 2025 · 6 min read
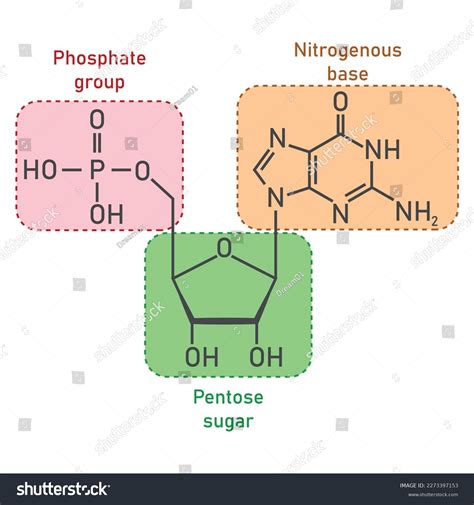
Table of Contents
What are the Components of a Nucleotide? A Deep Dive into the Building Blocks of Life
Nucleotides are fundamental building blocks of life, forming the basis of DNA and RNA, the genetic blueprints of all known organisms. Understanding their composition is crucial to comprehending the intricate mechanisms of heredity, gene expression, and cellular processes. This article provides a comprehensive overview of the components of a nucleotide, delving into their structure, function, and significance in biological systems.
The Tripartite Nature of Nucleotides: Sugar, Base, and Phosphate
A nucleotide is a remarkably simple yet incredibly versatile molecule, composed of three essential components:
-
A pentose sugar: This is a five-carbon sugar molecule, either ribose (in RNA) or deoxyribose (in DNA). The difference lies in the presence of a hydroxyl (-OH) group on the 2' carbon of ribose, which is absent in deoxyribose. This seemingly small difference has profound implications for the stability and function of the nucleic acids.
-
A nitrogenous base: This is a nitrogen-containing ring structure that is either a purine or a pyrimidine. Purines are larger, double-ring structures including adenine (A) and guanine (G). Pyrimidines are smaller, single-ring structures including cytosine (C), thymine (T) – found only in DNA – and uracil (U) – found only in RNA. These bases pair specifically in DNA (A with T, and G with C) and RNA (A with U, and G with C) via hydrogen bonds, forming the basis of the double helix structure in DNA and the secondary structures in RNA.
-
A phosphate group: This is a negatively charged molecule composed of a phosphorus atom bonded to four oxygen atoms (PO₄³⁻). The phosphate group imparts a negative charge to the nucleotide, influencing its interactions with other molecules and contributing to the overall stability of nucleic acids. The phosphate backbone also plays a crucial role in the energy transfer processes within cells, especially ATP (adenosine triphosphate).
Detailed Examination of Each Component
Let's explore each component in greater detail:
1. The Pentose Sugar: Ribose vs. Deoxyribose
The pentose sugar acts as the backbone for the nucleotide, providing the framework to which the base and phosphate group are attached. The difference between ribose and deoxyribose is critical:
-
Ribose (RNA): The presence of the 2'-hydroxyl group in ribose makes RNA more susceptible to hydrolysis (breakdown by water), making it less stable than DNA. This instability is, however, functionally important, as it allows RNA to undergo more rapid turnover, making it ideal for its roles in gene expression and regulation.
-
Deoxyribose (DNA): The absence of the 2'-hydroxyl group in deoxyribose increases the stability of DNA, crucial for its role in long-term storage of genetic information. This increased stability ensures the faithful transmission of genetic material across generations.
The specific arrangement of the carbon atoms in the sugar molecule is also crucial. The numbering of the carbons (1' to 5') dictates the point of attachment for the other components. The base attaches to the 1' carbon, and the phosphate group connects to the 5' carbon.
2. The Nitrogenous Bases: Purines and Pyrimidines
The nitrogenous bases are the information-carrying components of nucleotides. Their specific sequence dictates the genetic code, determining the amino acid sequence of proteins and ultimately the traits of an organism. The differing structures and properties of these bases are central to their function:
-
Purines (Adenine and Guanine): These double-ring structures are more structurally robust than pyrimidines. The specific arrangement of nitrogen and carbon atoms within the rings influences their ability to form hydrogen bonds with complementary bases. For instance, adenine's structure allows it to form two hydrogen bonds with thymine (in DNA) or uracil (in RNA).
-
Pyrimidines (Cytosine, Thymine, and Uracil): These single-ring structures are smaller and less complex than purines. Their structure allows for specific hydrogen bonding with complementary bases: Cytosine forms three hydrogen bonds with guanine, while thymine and uracil each form two hydrogen bonds with adenine. The difference between thymine and uracil, a methyl group, is significant for distinguishing DNA from RNA.
The nitrogenous bases absorb ultraviolet (UV) light at a specific wavelength, a property used in techniques like spectrophotometry to quantify nucleic acid concentrations.
3. The Phosphate Group: The Energetic and Structural Backbone
The phosphate group is not just a structural component; it plays a vital role in energy transfer and the overall stability of nucleic acids.
-
Energy Transfer: The phosphate bonds within nucleotides, particularly in ATP (adenosine triphosphate), are high-energy bonds. The hydrolysis of these bonds releases significant energy that drives numerous cellular processes, including muscle contraction, protein synthesis, and active transport. Other nucleotides like GTP (guanosine triphosphate) and CTP (cytidine triphosphate) also have energy transfer roles.
-
Structural Integrity: The phosphate group links the sugar molecules of adjacent nucleotides, forming the sugar-phosphate backbone of nucleic acids. This backbone provides structural stability and defines the directionality of the nucleic acid strand (5' to 3'). The negative charges on the phosphate groups repel each other, contributing to the overall stability of the double helix in DNA.
The phosphate group can exist as a mono-, di-, or triphosphate, affecting the nucleotide's energy potential and function. For example, ATP is a triphosphate, while AMP (adenosine monophosphate) is a monophosphate, and their different energy levels are important for cellular regulation.
Nucleotide Functions Beyond DNA and RNA
While nucleotides are best known for their roles in DNA and RNA, their functions extend far beyond these molecules:
-
Energy Carriers: ATP is the primary energy currency of cells, fueling a vast array of biological processes. GTP also plays a role in energy transfer and signal transduction pathways.
-
Enzyme Cofactors: Some nucleotides act as cofactors for enzymes, facilitating catalytic reactions. NAD+ (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide) are essential coenzymes involved in redox reactions in cellular respiration.
-
Cellular Signaling: Cyclic AMP (cAMP) is a crucial second messenger in signal transduction pathways, mediating the effects of hormones and other extracellular signals.
-
Structural Components: Nucleotides are involved in the structure of some coenzymes and metabolic intermediates.
Conclusion: The Remarkable Versatility of Nucleotides
In conclusion, nucleotides are far more than just the building blocks of DNA and RNA. Their tripartite structure—the pentose sugar, the nitrogenous base, and the phosphate group—allows for remarkable versatility in function. Understanding their composition and properties is essential for a thorough understanding of the fundamental processes of life, from heredity and gene expression to energy metabolism and cellular signaling. The seemingly simple molecular architecture of a nucleotide underpins the complexity and beauty of the biological world. Further research into nucleotide modifications, interactions, and their role in various cellular processes promises to reveal even deeper insights into the intricate mechanisms of life.
Latest Posts
Latest Posts
-
Which Layer Of Earth Is Hottest
Mar 10, 2025
-
What Are The Multiples Of 40
Mar 10, 2025
-
Lines Of Symmetry On An Octagon
Mar 10, 2025
-
The Junction Between Two Neurons Is Known As The
Mar 10, 2025
-
Units For Area Moment Of Inertia
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about What Are The Components Of A Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
