The State Of Matter In Which Water Is Compressible
Juapaving
Mar 31, 2025 · 5 min read
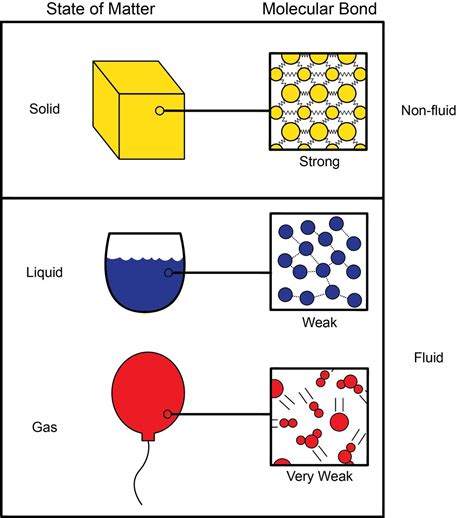
Table of Contents
The State of Matter in Which Water is Compressible: Exploring the Behavior of Water Under Pressure
Water, the elixir of life, is often perceived as incompressible. This is a reasonable approximation for everyday experiences – you can't squeeze a water balloon significantly smaller simply by applying hand pressure. However, the truth is more nuanced. Water is compressible, albeit to a much smaller extent than gases. Understanding the conditions under which water's compressibility becomes significant is crucial in various fields, from oceanography and geology to material science and engineering. This article delves into the complexities of water's compressibility, exploring the underlying physics and its implications across different disciplines.
Understanding Compressibility: Beyond the Everyday
Compressibility, in simple terms, refers to the ability of a substance to decrease in volume when subjected to external pressure. Gases are highly compressible due to the large spaces between their molecules. Liquids, including water, are far less compressible because their molecules are much closer together. However, even in liquids, the intermolecular forces are not completely rigid, allowing for some degree of volume reduction under sufficient pressure.
The compressibility of a substance is often quantified using the isothermal compressibility, denoted by β, which represents the fractional change in volume per unit change in pressure at a constant temperature:
β = - (1/V) (∂V/∂P)<sub>T</sub>
where:
- V is the volume
- P is the pressure
- T is the temperature
- ∂V/∂P represents the partial derivative of volume with respect to pressure
A lower value of β indicates lower compressibility. For water at room temperature and atmospheric pressure, the isothermal compressibility is relatively low, around 4.5 x 10<sup>-10</sup> Pa<sup>-1</sup>. This seemingly small number reflects the fact that significant pressure is required to induce noticeable volume changes in water.
Factors Influencing Water's Compressibility
Several factors influence the compressibility of water:
-
Temperature: Water's compressibility generally decreases as temperature increases. At higher temperatures, the molecules possess more kinetic energy, making it more difficult to reduce their volume.
-
Pressure: While the relationship isn't perfectly linear, higher pressures generally lead to greater compression. The effect is more pronounced at higher pressures.
-
Presence of Dissolved Substances: The presence of dissolved salts and other substances in water can slightly alter its compressibility. This is because the dissolved substances interact with water molecules, influencing the intermolecular forces and hence the resistance to compression.
-
Phase: The compressibility of water is significantly different in its different phases (solid, liquid, gas). Ice, for example, is less compressible than liquid water. Water vapor, being a gas, is far more compressible.
The Significance of Water's Compressibility in Different Fields
While water's compressibility is relatively low, it is not negligible, particularly in situations involving high pressures or large volumes of water. Its significance extends to various fields:
1. Oceanography and Marine Geophysics:
The immense pressure exerted at significant depths in the ocean plays a critical role in shaping the properties of seawater. The compressibility of water affects:
-
Sound Velocity: The speed of sound in seawater increases with increasing pressure due to water's compressibility. This is crucial for underwater acoustics and sonar technology.
-
Ocean Circulation: Water's compressibility influences density gradients in the ocean, contributing to the formation of deep ocean currents. As water is compressed, its density increases, driving thermohaline circulation patterns.
-
Seafloor Mapping: Understanding water's compressibility is essential for interpreting data from sonar and other geophysical tools used to map the ocean floor and study its geological structures.
2. Hydrology and Groundwater Studies:
Water's compressibility is relevant in analyzing:
-
Aquifer Compaction: Groundwater aquifers consist of porous geological formations holding water. When water is extracted from an aquifer, the reduced water pressure can cause the aquifer to compact, leading to land subsidence. Accurate models of aquifer behavior require considering the compressibility of both the water and the rock matrix.
-
Water Well Design: Understanding the compressibility of water is crucial for designing efficient and sustainable water well systems. This is important for managing groundwater resources and preventing issues such as well collapse or water contamination.
3. Material Science and Engineering:
Water's compressibility impacts the properties of materials that interact with water under pressure:
-
Hydraulic Systems: In hydraulic machinery and systems, water is often used as a working fluid under high pressure. The compressibility of water needs to be considered in designing these systems to ensure their effective and safe operation. Ignoring compressibility can lead to inaccurate pressure calculations and system malfunctions.
-
High-Pressure Applications: In many high-pressure industrial processes, understanding the behavior of water under pressure is critical. This includes applications such as high-pressure cleaning, water jet cutting, and deep-sea exploration equipment.
4. Meteorology and Climatology:
Although water's compressibility plays a less dominant role than temperature and pressure in atmospheric dynamics, it still contributes to:
-
Cloud Formation: The compressibility of water vapor in clouds affects the equilibrium between condensation and evaporation processes.
-
Hurricane Intensity: Water's compressibility can subtly influence the dynamics of hurricanes, particularly at very high pressures near the eye.
Advanced Concepts and Research
The study of water's compressibility extends beyond simple isothermal compressibility. Researchers investigate:
-
Adiabatic Compressibility: This refers to compressibility at constant entropy, relevant to processes occurring without heat exchange, such as sound propagation in water.
-
Equation of State: Developing accurate equations of state for water, which relate its pressure, temperature, and volume, is an active area of research. These equations are crucial for modeling water's behavior under extreme conditions of pressure and temperature.
-
Molecular Dynamics Simulations: Computer simulations using molecular dynamics techniques allow researchers to study the microscopic behavior of water molecules under pressure, providing insights into the mechanisms of compression.
Conclusion
Water's compressibility, while often overlooked in everyday contexts, is a significant factor in many scientific and engineering disciplines. From the depths of the ocean to the design of hydraulic systems, understanding the conditions under which water's compressibility becomes relevant is crucial for accurate modeling, efficient design, and safe operation. As research progresses and computational tools improve, our understanding of this fundamental property of water will continue to evolve, leading to advancements in various fields. The seemingly simple substance of water continues to reveal its complex and fascinating behavior under pressure, highlighting the importance of continuous investigation and exploration.
Latest Posts
Latest Posts
-
Magnetic Field From A Current Loop
Apr 02, 2025
-
Collection Of Nerve Cell Bodies Outside The Cns
Apr 02, 2025
-
What Do Nitrification And Denitrification Have In Common
Apr 02, 2025
-
What Is The Lowest Common Multiple Of 3 And 7
Apr 02, 2025
-
How To Find A Supplementary Angle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about The State Of Matter In Which Water Is Compressible . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
