The Smallest Particle Of An Element
Juapaving
Mar 26, 2025 · 6 min read
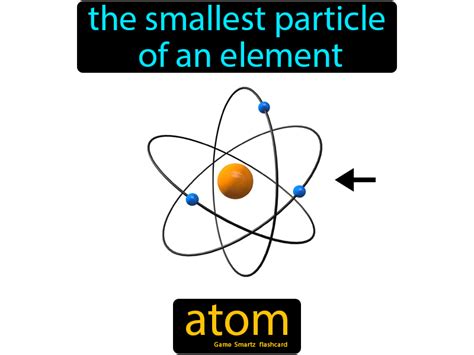
Table of Contents
Delving into the Atom: Exploring the Smallest Particle of an Element
The quest to understand the fundamental building blocks of matter has captivated scientists for centuries. From the ancient Greek philosophers pondering atoms to modern physicists unraveling the complexities of quantum mechanics, the journey has been one of relentless discovery. This article delves deep into the fascinating world of the atom, exploring what constitutes the smallest particle of an element and the intricacies of its subatomic components.
The Atom: A Brief History
The concept of the atom, meaning "indivisible" in Greek, originated with Leucippus and Democritus in the 5th century BC. However, this remained a philosophical idea until the late 19th and early 20th centuries when scientific experimentation provided tangible evidence. John Dalton's atomic theory (1803) proposed that all matter is composed of indivisible atoms, a significant leap forward in our understanding.
However, subsequent discoveries shattered the notion of the atom as an indivisible unit. J.J. Thomson's cathode ray experiments (1897) revealed the existence of electrons, negatively charged subatomic particles. This led to the plum pudding model, picturing the atom as a positively charged sphere with negatively charged electrons embedded within it.
Ernest Rutherford's gold foil experiment (1911) revolutionized our understanding. By bombarding a thin gold foil with alpha particles, he observed that most particles passed through, but a few were deflected at large angles. This led to the nuclear model, proposing that the atom consists of a tiny, dense, positively charged nucleus at the center, surrounded by orbiting electrons.
Niels Bohr refined the model further (1913) by introducing quantized energy levels for electrons, explaining the stability of the atom and the discrete nature of spectral lines. This Bohr model, though eventually superseded, represented a crucial step toward a more accurate picture.
Subatomic Particles: Exploring the Nucleus and Beyond
The atom is no longer considered indivisible. Instead, it's composed of three fundamental subatomic particles:
1. Protons: The Positively Charged Heart
Protons reside within the nucleus and carry a positive electrical charge, equal in magnitude to the electron's negative charge. The number of protons in an atom's nucleus defines its atomic number and determines the element. For instance, an atom with one proton is hydrogen, two protons is helium, and so on. Protons are significantly heavier than electrons, contributing most of the atom's mass.
Key characteristics of protons:
- Charge: +1
- Mass: Approximately 1.673 x 10<sup>-27</sup> kg (1836 times the mass of an electron)
- Location: Nucleus
- Composition: Composed of three quarks (two up quarks and one down quark)
2. Neutrons: The Neutral Partners
Neutrons, as their name suggests, carry no electrical charge. They reside in the nucleus alongside protons, contributing significantly to the atom's mass. The number of neutrons in an atom can vary, leading to isotopes of the same element. Isotopes have the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are radioactive, undergoing decay to achieve stability.
Key characteristics of neutrons:
- Charge: 0
- Mass: Approximately 1.675 x 10<sup>-27</sup> kg (slightly heavier than a proton)
- Location: Nucleus
- Composition: Composed of three quarks (one up quark and two down quarks)
3. Electrons: The Orbiting Negatives
Electrons are negatively charged subatomic particles that orbit the nucleus at significant distances. Their movement is governed by the laws of quantum mechanics, making precise location determination impossible. Instead, their positions are described by probability distributions, known as orbitals. The number of electrons in a neutral atom equals the number of protons, ensuring an overall neutral charge.
Key characteristics of electrons:
- Charge: -1
- Mass: Approximately 9.109 x 10<sup>-31</sup> kg (much lighter than protons and neutrons)
- Location: Orbitals surrounding the nucleus
- Wave-particle duality: Electrons exhibit both wave-like and particle-like properties.
Beyond the Standard Model: Quarks and Leptons
While protons, neutrons, and electrons were once considered fundamental particles, further research revealed that they are composed of even smaller constituents. This deeper understanding falls under the realm of the Standard Model of particle physics.
Quarks: The Building Blocks of Protons and Neutrons
Protons and neutrons are made up of fundamental particles called quarks. There are six types (or "flavors") of quarks: up, down, charm, strange, top, and bottom. Each quark possesses a fractional electric charge (+2/3 or -1/3). Protons are composed of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks. The strong force, mediated by gluons, holds these quarks together within protons and neutrons.
Leptons: A Different Class of Fundamental Particles
Electrons belong to a family of fundamental particles called leptons. Leptons are fundamental particles that do not experience the strong force. Besides electrons, other leptons include muons and tau particles, along with their associated neutrinos. These particles are involved in weak interactions, responsible for radioactive decay.
Isotopes and Their Significance
As mentioned earlier, isotopes are atoms of the same element that have the same number of protons but differ in the number of neutrons. This difference affects the atom's mass but not its chemical properties. Many elements exist as mixtures of isotopes, with their relative abundances determining the element's average atomic mass.
Isotopes play crucial roles in various fields:
- Radioactive dating: Radioactive isotopes, which undergo decay at a known rate, are used to determine the age of artifacts and geological formations. Carbon-14 dating is a prime example.
- Medical imaging and treatment: Radioactive isotopes are employed in medical procedures like PET scans and radiotherapy.
- Industrial applications: Isotopes find applications in various industrial processes, such as tracing materials and gauging thicknesses.
The Future of Atomic Research
Our understanding of the atom is continuously evolving. Research in particle physics continues to delve into the intricacies of subatomic particles, exploring phenomena like dark matter and dark energy. Advanced technologies, such as particle accelerators, enable scientists to probe deeper into the structure of matter, pushing the boundaries of our knowledge. The exploration of the smallest particle of an element is an ongoing journey, with future discoveries promising to further refine our understanding of the universe's fundamental building blocks.
Conclusion: A Journey of Discovery
The journey from the ancient concept of the indivisible atom to the complex Standard Model of particle physics highlights the remarkable progress in our understanding of matter. While the atom is no longer considered indivisible, its components – protons, neutrons, and electrons – represent fundamental building blocks with profound implications across various scientific disciplines. Continuing research promises further revelations, deepening our understanding of the universe and its fundamental constituents. The quest to fully understand the smallest particle of an element continues, driving scientific advancements and shaping our perception of reality itself. The study of atomic structure and its subatomic particles remains a vibrant and ever-evolving field of scientific endeavor, with countless discoveries yet to be made.
Latest Posts
Latest Posts
-
What Is The Gcf Of 18 And 12
Mar 29, 2025
-
What Is Commonly Called The King Of Spices
Mar 29, 2025
-
How To Find Inverse Of A Relation
Mar 29, 2025
-
What Is The Lowest Common Multiple Of 2 And 3
Mar 29, 2025
-
What Is The Electron Configuration Of Rubidium
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about The Smallest Particle Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
