What Is The Electron Configuration Of Rubidium
Juapaving
Mar 29, 2025 · 5 min read
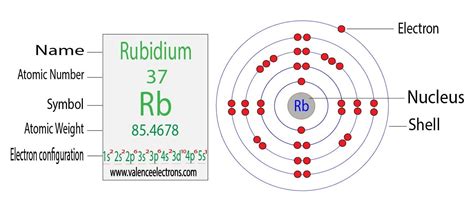
Table of Contents
What is the Electron Configuration of Rubidium? A Deep Dive into Atomic Structure
Rubidium, a fascinating alkali metal, holds a unique place in the periodic table. Understanding its electron configuration is key to unlocking its chemical properties and behavior. This comprehensive guide will delve into the intricacies of rubidium's electron arrangement, exploring the underlying principles of electron shells, subshells, orbitals, and the significance of its position in the periodic table. We'll also touch upon the implications of its electron configuration for its reactivity and applications.
Understanding Electron Configuration
Before we dive into rubidium's specific electron configuration, let's establish a foundational understanding of what electron configuration represents. An electron configuration is a notation that describes the arrangement of electrons in the various energy levels and sublevels within an atom. It dictates how an atom will interact with other atoms, forming molecules and compounds.
The fundamental principles underpinning electron configuration are based on:
- Quantum Mechanics: The behavior of electrons within an atom is governed by the principles of quantum mechanics, which dictates that electrons occupy specific energy levels and orbitals.
- Aufbau Principle: This principle states that electrons first fill the lowest energy levels available before moving to higher energy levels. It's like filling a building from the ground floor up.
- Pauli Exclusion Principle: This principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms). This essentially limits the number of electrons that can occupy a single orbital to two, with opposite spins.
- Hund's Rule: This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion.
These principles guide us in predicting the electron configuration of any element, including rubidium.
Determining Rubidium's Electron Configuration
Rubidium (Rb) has an atomic number of 37, meaning it possesses 37 protons and, in a neutral atom, 37 electrons. To determine its electron configuration, we'll follow the Aufbau principle, filling the orbitals in order of increasing energy.
The order of filling orbitals is typically represented by the following sequence: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... and so on.
Let's fill the orbitals for rubidium:
- 1s²: The first energy level (n=1) contains the 1s subshell, which can hold a maximum of two electrons.
- 2s²: The second energy level (n=2) contains the 2s subshell, also holding two electrons.
- 2p⁶: The 2p subshell has three orbitals, each holding two electrons, for a total of six electrons.
- 3s²: The 3s subshell holds two electrons.
- 3p⁶: The 3p subshell holds six electrons.
- 4s²: The 4s subshell holds two electrons.
- 3d¹⁰: The 3d subshell has five orbitals, holding a total of ten electrons.
- 4p⁶: The 4p subshell holds six electrons.
- 5s¹: Finally, the remaining electron occupies the 5s subshell.
Therefore, the complete electron configuration of rubidium is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹.
This can also be written in a shorthand notation using the noble gas configuration. Since krypton (Kr) has the electron configuration 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶, we can write rubidium's configuration as: [Kr]5s¹. This shorthand notation is more concise and highlights the valence electron, which plays a crucial role in rubidium's chemical reactivity.
The Significance of the 5s¹ Electron
The single electron in the 5s orbital is the valence electron of rubidium. This electron is relatively loosely held by the nucleus and is readily involved in chemical bonding. This is the primary reason why rubidium is highly reactive. Its tendency to lose this valence electron to achieve a stable noble gas configuration (like krypton) drives its chemical behavior.
Reactivity and Chemical Properties
The presence of this lone valence electron dictates rubidium's properties:
- High Reactivity: Rubidium readily loses its valence electron to form a +1 ion (Rb⁺). This makes it highly reactive, particularly with water and other oxidizing agents. Reactions with water are often vigorous and exothermic.
- Alkali Metal Properties: Its position in Group 1 of the periodic table, the alkali metals, reflects its properties: low ionization energy, low electronegativity, and a tendency to form ionic compounds.
- Formation of Ionic Compounds: Rubidium readily forms ionic compounds with nonmetals, transferring its valence electron to achieve a stable octet.
- Low Melting and Boiling Points: Due to weak metallic bonding, rubidium possesses low melting and boiling points compared to transition metals.
Applications of Rubidium
Rubidium's unique properties have led to its use in various applications:
- Atomic Clocks: Rubidium's specific atomic transitions make it suitable for use in highly accurate atomic clocks.
- Medical Imaging: Certain rubidium isotopes are used in medical imaging techniques.
- Photoelectric Cells: Its sensitivity to light makes it useful in photoelectric cells.
- Research Applications: Rubidium is used extensively in various scientific research areas due to its specific spectral properties and chemical reactivity.
Conclusion: Understanding Rubidium's Electron Configuration
Understanding the electron configuration of rubidium, specifically its [Kr]5s¹ configuration and the significance of its single valence electron, is crucial for grasping its chemical behavior and applications. This configuration dictates its high reactivity, its typical alkali metal properties, and its involvement in diverse technological and scientific applications. The principles of quantum mechanics and the rules governing electron filling provide a fundamental framework for predicting and interpreting the behavior of this fascinating element. Further research into the specific interactions of its valence electron can lead to a more profound understanding of its potential applications in various fields. The continued study of rubidium's electron configuration will undoubtedly contribute to advancements in technology and scientific understanding.
Latest Posts
Latest Posts
-
What Is The Lcm Of 9 12 15
Apr 01, 2025
-
Five Letter Word Ends With Er
Apr 01, 2025
-
What Is The Correct Order Of Photosynthesis
Apr 01, 2025
-
Give An Example Of A Multicellular Organism
Apr 01, 2025
-
17 Out Of 25 As A Percentage
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Rubidium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
