The Proper Electron-dot Symbol For Aluminum Is
Juapaving
Apr 01, 2025 · 5 min read
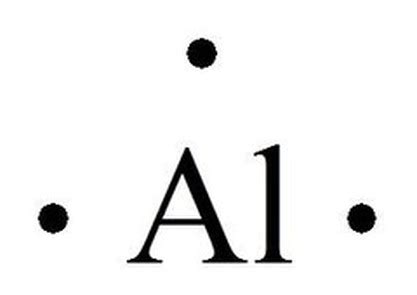
Table of Contents
The Proper Electron-Dot Symbol for Aluminum is... Al with Three Dots! A Deep Dive into Electron Dot Structures and Aluminum's Valence Electrons
Understanding electron dot symbols, also known as Lewis dot structures, is fundamental to grasping chemical bonding and the behavior of elements. This article will delve deep into the proper electron-dot symbol for aluminum, explaining the underlying principles and providing a solid foundation for further exploration of chemical concepts. We'll explore not just the how, but also the why, solidifying your understanding of aluminum's reactivity and its place in the periodic table.
Understanding Electron Dot Structures: A Visual Representation of Valence Electrons
Electron dot structures are simplified diagrams that represent the valence electrons of an atom. Valence electrons are the electrons located in the outermost shell (energy level) of an atom. These electrons are crucial because they participate in chemical bonding, determining an element's reactivity and the types of compounds it can form. The electron dot structure provides a quick visual way to understand an element's bonding capacity.
Key Components of an Electron Dot Structure:
- The Element Symbol: The central part of the structure is the element's symbol, as found on the periodic table (e.g., Al for aluminum, O for oxygen, etc.).
- Valence Electrons: These are represented by dots placed around the element symbol. Each dot represents a single valence electron. Dots are placed individually around the symbol until all valence electrons are accounted for, then pairing begins.
Determining the Number of Valence Electrons: The Periodic Table as Your Guide
The number of valence electrons an atom possesses is easily determined using the element's position on the periodic table. For the main group elements (groups 1-18), the group number (using the American system) directly corresponds to the number of valence electrons.
- Group 1 (Alkali Metals): 1 valence electron
- Group 2 (Alkaline Earth Metals): 2 valence electrons
- Group 13 (Boron Group): 3 valence electrons
- Group 14 (Carbon Group): 4 valence electrons
- Group 15 (Pnictogens): 5 valence electrons
- Group 16 (Chalcogens): 6 valence electrons
- Group 17 (Halogens): 7 valence electrons
- Group 18 (Noble Gases): 8 valence electrons (except helium, which has 2)
Aluminum's Position and its Valence Electrons
Aluminum (Al) resides in Group 13 of the periodic table. Therefore, it possesses three valence electrons.
Constructing the Electron Dot Symbol for Aluminum
Now that we know aluminum has three valence electrons, we can construct its electron dot symbol:
Al • • •
The "Al" represents the aluminum atom, and the three dots surrounding it signify its three valence electrons. Note that the dots are initially placed individually before pairing.
Why this is the Correct Representation:
This representation accurately reflects aluminum's electronic configuration. Aluminum's electron configuration is 1s²2s²2p⁶3s²3p¹. The 3s² and 3p¹ electrons are the valence electrons, totaling three. The 1s², 2s², and 2p⁶ electrons are core electrons and are not involved in chemical bonding.
The Significance of Aluminum's Three Valence Electrons
The presence of three valence electrons explains aluminum's chemical behavior. Aluminum tends to lose these three electrons to achieve a stable octet (eight electrons in its outermost shell) configuration, mimicking the noble gases. This electron loss results in the formation of a +3 ion (Al³⁺). This characteristic is crucial in understanding:
- Aluminum's Reactivity: Aluminum readily reacts with non-metals, particularly oxygen and halogens, to form stable compounds. This reactivity stems from its eagerness to lose its three valence electrons to achieve a stable electron configuration.
- Formation of Ionic Compounds: Aluminum readily forms ionic compounds by transferring its three valence electrons to a more electronegative atom (an atom with a stronger tendency to attract electrons). Examples include aluminum oxide (Al₂O₃) and aluminum chloride (AlCl₃).
- Aluminum's Metallic Properties: The ease with which aluminum loses its valence electrons contributes to its excellent electrical and thermal conductivity, a typical characteristic of metals.
Comparing Aluminum's Electron Dot Structure to Other Elements:
Let's compare Aluminum's electron dot structure to elements in the same period and group to highlight the trends in valence electrons and reactivity.
Group 13 (Boron Group):
- Boron (B): •B• (3 valence electrons) – Boron, like aluminum, is also a reactive element, though less so.
- Gallium (Ga): •Ga• • • (3 valence electrons) – Shares similar properties with Aluminum.
- Indium (In): •In• • • (3 valence electrons) – Shares similar properties with Aluminum.
- Thallium (Tl): •Tl• • • (3 valence electrons) – Shares similar properties with Aluminum.
Period 3:
- Sodium (Na): Na• (1 valence electron) – Highly reactive alkali metal.
- Magnesium (Mg): Mg•• (2 valence electrons) – Reactive alkaline earth metal.
- Silicon (Si): •Si• • • • (4 valence electrons) – Forms covalent bonds.
- Phosphorus (P): •P• • • • • (5 valence electrons) – Forms covalent bonds.
- Sulfur (S): •S•• • • • (6 valence electrons) – Forms covalent bonds.
- Chlorine (Cl): •Cl•• • • • • (7 valence electrons) – Highly reactive halogen.
- Argon (Ar): :Ar: (8 valence electrons) – Inert noble gas.
This comparison demonstrates how the number of valence electrons directly impacts an element's reactivity and bonding characteristics. Aluminum, with its three valence electrons, occupies a key position, exhibiting metallic properties and readily forming ionic compounds.
Beyond the Basics: Advanced Applications of Electron Dot Structures
While simple, electron dot structures are powerful tools extending beyond basic understanding. They are used in visualizing:
- Covalent Bonding: While aluminum primarily forms ionic bonds, electron dot structures can illustrate how it participates in some covalent bonding situations in complex compounds. The sharing of electron pairs is easily shown.
- Coordinate Covalent Bonding: Understanding dative bonds and coordination complexes often involves using electron dot structures.
- Resonance Structures: For some molecules and ions, multiple electron dot structures are possible, representing resonance. Aluminum's participation in such structures, though less common than in elements like carbon or nitrogen, is also possible in complex compounds.
Conclusion: Mastering Aluminum's Electron Dot Structure
The proper electron-dot symbol for aluminum is Al • • •, accurately representing its three valence electrons. Understanding this simple structure provides a crucial foundation for comprehending aluminum's reactivity, bonding behavior, and its place within the broader context of chemical principles. By mastering the construction and interpretation of electron dot structures, you gain a valuable tool for analyzing chemical interactions and predicting the properties of compounds. This knowledge extends far beyond simple diagrams, offering a window into the fundamental forces shaping the world around us. Continued exploration of these concepts will build a stronger, more intuitive understanding of chemistry.
Latest Posts
Latest Posts
-
How Tall Is 33 Inches In Feet
Apr 02, 2025
-
Which State Of Matter Has No Definite Shape Or Volume
Apr 02, 2025
-
Whats The Square Root Of 100
Apr 02, 2025
-
Plant Is Where Photosynthesis Takes Place
Apr 02, 2025
-
12 Cm Is How Many Inches
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about The Proper Electron-dot Symbol For Aluminum Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
