The Principle Of Constant Proportions States That:
Juapaving
Mar 30, 2025 · 6 min read
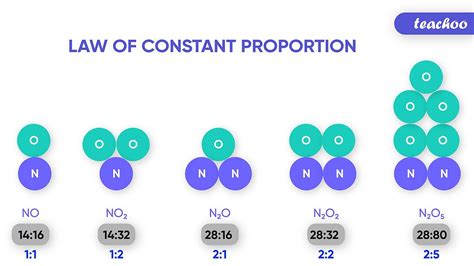
Table of Contents
The Principle of Constant Proportions: A Deep Dive into the Law of Definite Proportions
The principle of constant proportions, also known as the law of definite proportions or Proust's law, is a fundamental concept in chemistry. It dictates that a chemical compound always contains exactly the same proportion of elements by mass. This seemingly simple statement underpins much of our understanding of chemical reactions, stoichiometry, and the very nature of matter itself. Let's delve into the intricacies of this principle, exploring its historical context, experimental verification, exceptions, and broader implications.
A Historical Perspective: The Genesis of Proust's Law
Before the advent of sophisticated analytical techniques, the composition of chemical compounds was often a matter of guesswork. Different chemists might prepare the same compound, yet obtain varying results due to impurities or inconsistencies in their methods. This ambiguity fueled much debate within the scientific community.
Enter Joseph Proust, a French chemist who, in the late 18th and early 19th centuries, meticulously studied the composition of various compounds. Through painstaking experimentation, particularly with copper carbonate, Proust consistently found that regardless of the source or method of preparation, the ratio of copper, carbon, and oxygen remained remarkably constant. This led him to formulate the principle of constant proportions, published between 1797 and 1808. His work directly challenged the prevailing belief, championed by Claude Louis Berthollet, that the proportions of elements in a compound could vary within certain limits. The ensuing debate played a crucial role in solidifying the foundation of modern chemistry.
Experimental Evidence and Verification: Proving the Principle
The principle of constant proportions isn't merely a theoretical postulate; it's been verified through countless experiments over the years. Modern analytical techniques like mass spectrometry and atomic absorption spectroscopy allow for precise determination of elemental composition, consistently confirming Proust's findings. For instance, consider water (H₂O): no matter whether the water is extracted from a glacier, a rain cloud, or a laboratory synthesis, the mass ratio of hydrogen to oxygen will always be approximately 1:8. This consistency extends to a vast array of compounds, demonstrating the universality of the principle.
The experimental verification involves several steps:
- Preparation of the Compound: The compound in question needs to be carefully prepared using well-defined procedures to ensure purity.
- Purification of the Compound: Impurities can significantly alter the observed proportions. Therefore, purification techniques are crucial to obtaining accurate results.
- Elemental Analysis: Sophisticated analytical methods are employed to determine the exact mass of each element present in a known mass of the compound.
- Calculation of Mass Ratios: The mass ratios of the constituent elements are calculated and compared across multiple samples prepared under different conditions. Consistency in these ratios validates the principle.
Understanding the Atomic Basis: Linking to Dalton's Atomic Theory
The principle of constant proportions finds its elegant explanation in Dalton's atomic theory. Dalton proposed that matter consists of indivisible atoms, each element having a unique type of atom with a characteristic mass. Chemical compounds are formed by the combination of atoms of different elements in specific, whole-number ratios. This directly explains why the mass ratios of elements in a compound are constant. For example, in the formation of water, two hydrogen atoms combine with one oxygen atom, resulting in a fixed mass ratio.
This link between Dalton's atomic theory and Proust's law is a cornerstone of modern chemistry. It underscores the discrete nature of matter and the importance of stoichiometry – the quantitative relationships between reactants and products in chemical reactions.
Exceptions and Limitations: Nuances of the Principle
While the principle of constant proportions holds true for most compounds, there are some notable exceptions, predominantly concerning non-stoichiometric compounds or berthollides. These compounds have variable compositions, often due to defects in their crystal structures. Examples include many metal oxides and certain interstitial compounds. In these instances, the ratio of elements isn't strictly fixed but varies within a certain range.
However, it's important to note that even these exceptions don't invalidate the fundamental principle. Rather, they highlight the complexities of solid-state chemistry and the limitations of applying a strictly defined law to all material systems. The variations in composition often arise from crystallographic defects, differences in oxidation states, or the presence of impurities. Careful consideration of the specific material and its structural features is crucial for understanding apparent deviations.
Applications and Importance: The Broad Reach of Proust's Law
The principle of constant proportions has profound implications across diverse areas of chemistry and related fields. Here are some key applications:
- Stoichiometric Calculations: The principle is fundamental to stoichiometric calculations, allowing us to predict the quantities of reactants and products in chemical reactions based on the known composition of compounds. This is crucial in various industrial processes, pharmaceutical development, and environmental chemistry.
- Chemical Analysis: The constant composition of compounds enables us to determine the purity of substances and quantify their constituents through analytical techniques like titration and gravimetric analysis.
- Material Science: Understanding the constant proportions in materials allows for the design and synthesis of materials with specific properties. The precise control over elemental ratios is key to developing novel materials with tailored characteristics.
- Forensic Science: The principle can be applied to analyze evidence samples, helping to identify substances and match them to their sources. This is especially important in drug identification and trace evidence analysis.
Beyond the Basics: Delving into Isotopes and Isotopic Abundance
While the principle of constant proportions holds for the mass ratios of elements, it's crucial to acknowledge the existence of isotopes. Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This means they have the same atomic number but a different mass number.
The isotopic abundance of an element – the relative proportion of each isotope in a naturally occurring sample – varies slightly depending on the source. Therefore, while the mass ratio of elements in a compound remains constant, the absolute masses of the constituent atoms may vary slightly due to isotopic variations. However, these variations are usually minor and don't significantly affect the overall principle. For highly precise work, however, consideration of isotopic abundances is necessary for accurate calculations.
Conclusion: A Cornerstone of Chemical Understanding
The principle of constant proportions, despite its seemingly straightforward nature, is a cornerstone of chemical understanding. It stands as a testament to the meticulous work of Joseph Proust and underpins much of the quantitative aspects of chemistry. While exceptions exist, the principle provides a powerful framework for analyzing and predicting the behavior of matter. Its enduring relevance underscores its importance in various scientific disciplines and highlights its ongoing contribution to advancements in diverse fields. Understanding this principle is not merely about memorizing a law but appreciating the profound implications it holds for our understanding of the world around us. From industrial processes to forensic investigations, the constant proportions of elements in compounds remain a critical factor in a wide variety of applications, solidifying its place as a fundamental law of chemistry.
Latest Posts
Latest Posts
-
Which Elements Had A Filled Outermost Shell
Apr 01, 2025
-
Is Potassium A Metal Or Nonmetal
Apr 01, 2025
-
How Many Solutions Does This Equation Have
Apr 01, 2025
-
Is Cellular Respiration Exergonic Or Endergonic
Apr 01, 2025
-
What Is The Gcf Of 12 And 30
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Principle Of Constant Proportions States That: . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
