Which Elements Had A Filled Outermost Shell
Juapaving
Apr 01, 2025 · 5 min read
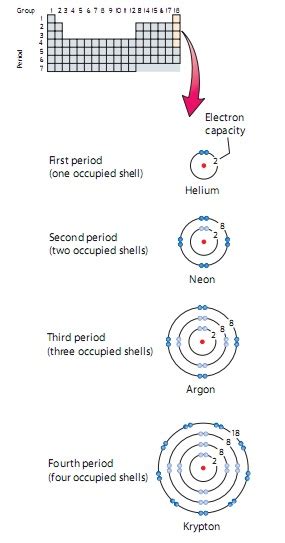
Table of Contents
Which Elements Have a Filled Outermost Shell? Understanding Noble Gases and Electron Configurations
The periodic table is a marvel of organization, neatly arranging elements based on their atomic structure and properties. One of the most fundamental concepts in understanding elemental behavior is electron configuration – the arrangement of electrons in energy levels or shells surrounding the atom's nucleus. A key characteristic that dramatically influences an element's reactivity is whether its outermost shell, also known as the valence shell, is filled or not. This article delves deep into which elements boast a completely filled outermost shell, exploring their unique properties and the implications of this stable electron configuration.
The Significance of a Filled Outermost Shell
Atoms strive for stability, and a filled outermost shell represents the ultimate state of stability for most atoms. This is because a filled valence shell signifies a complete octet (eight electrons) for most elements, or a duet (two electrons) for the very lightest elements, like Helium. This configuration minimizes the atom's potential energy, making it less likely to react with other atoms to gain or lose electrons.
Elements with a complete valence shell are famously unreactive, showing little tendency to form chemical bonds. This characteristic is crucial to understanding their behavior in various contexts, from their existence in nature to their industrial applications.
The Noble Gases: The Quintessential Examples
The most prominent examples of elements with a completely filled outermost shell are the noble gases. Located in Group 18 (or VIIIA) of the periodic table, these elements – Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn), and Oganesson (Og) – are renowned for their exceptional inertness.
Helium (He): The Lightweight Champion
Helium, with only two electrons filling its 1s orbital (a duet), represents the simplest case of a filled outermost shell. This exceptionally stable configuration accounts for its inertness and low reactivity. Its lightness makes it ideal for applications like balloons and cryogenics.
Neon (Ne), Argon (Ar), and the Rest: The Octet Rule in Action
Neon, argon, and the other noble gases, excluding Helium, follow the octet rule, possessing eight electrons in their outermost shell. This complete octet renders them incredibly stable and unreactive under normal conditions. These gases are found in trace amounts in the atmosphere and have various uses, including lighting (neon signs) and shielding in specialized processes.
Understanding Electron Configuration and Valence Shells
To fully grasp why noble gases have filled outermost shells, it's crucial to understand the concept of electron configuration. Electrons occupy specific energy levels or shells around the nucleus. These shells are numbered, with the innermost shell (n=1) having the lowest energy. Each shell can accommodate a specific maximum number of electrons:
- Shell 1 (n=1): Maximum 2 electrons
- Shell 2 (n=2): Maximum 8 electrons
- Shell 3 (n=3): Maximum 18 electrons
- Shell 4 (n=4): Maximum 32 electrons
and so on. The outermost shell is the valence shell, and the electrons in this shell are the valence electrons. The number of valence electrons significantly determines an element's chemical behavior.
Subshells and Orbitals: A Deeper Dive
Within each shell, there are subshells (s, p, d, f), which are further divided into orbitals. Orbitals can hold a maximum of two electrons each. The filling of these subshells follows specific rules, including the Aufbau principle (electrons fill lower energy levels first) and Hund's rule (electrons fill orbitals individually before pairing up).
For example, Neon (Ne) has an atomic number of 10, meaning it has 10 electrons. Its electron configuration is 1s²2s²2p⁶. The outermost shell (n=2) is completely filled with 8 electrons (2 in the 2s subshell and 6 in the 2p subshell), hence its inert nature.
Exceptions and Nuances: Beyond the Simple Octet Rule
While the octet rule serves as a useful guideline, there are exceptions, especially among heavier elements. Some elements can achieve stability with less than or more than eight valence electrons.
Expanded Octet: Beyond Eight Electrons
Elements in the third period and beyond can sometimes accommodate more than eight electrons in their valence shell. This is because they have access to d orbitals, which can participate in bonding. For instance, phosphorus pentachloride (PCl5) exhibits an expanded octet around phosphorus.
Incomplete Octet: Stability with Fewer than Eight Electrons
Some elements, particularly those in the second period, can be stable with fewer than eight valence electrons. Boron, for example, often forms compounds with only six valence electrons.
Applications of Elements with Filled Outermost Shells
The unique properties of noble gases, stemming from their filled outermost shells, make them indispensable in various applications:
Lighting: Neon Signs and Beyond
Neon's distinctive glow in neon signs is a classic example. Other noble gases like argon, krypton, and xenon are also used in lighting applications due to their unique spectral emissions.
Welding and Metallurgy: Shielding Gases
Argon and helium are commonly employed as shielding gases in welding and metallurgical processes to prevent oxidation and contamination.
Cryogenics: Cooling and Preservation
Helium's extremely low boiling point makes it crucial in cryogenics, enabling the cooling of superconducting magnets and preservation of biological samples.
Medical Imaging: Xenon and MRI
Xenon is used as a contrast agent in medical imaging techniques such as magnetic resonance imaging (MRI).
Nuclear Medicine: Radon (with Cautions)
Radon, despite its radioactivity, finds application in certain radiotherapy techniques, highlighting the complex uses of even less-than-ideal elements.
Conclusion: The Importance of Electron Configuration
The presence or absence of a filled outermost shell significantly impacts an element's chemical reactivity and its applications. Understanding electron configuration is paramount for predicting and interpreting elemental behavior. While noble gases epitomize the stability associated with a complete valence shell, exceptions and nuances exist, adding to the richness and complexity of chemical interactions. The study of elements and their electron configurations forms the bedrock of chemistry and continues to inform groundbreaking discoveries in various scientific fields.
Latest Posts
Latest Posts
-
What Monument In India Is Made Of Metamorphic Rock
Apr 02, 2025
-
65 Inches In Feet And Inches
Apr 02, 2025
-
Why Are Fossils Found In Sedimentary Rocks
Apr 02, 2025
-
Why Are Fossil Fuels Considered Nonrenewable
Apr 02, 2025
-
What Is The Difference Between Purines And Pyrimidines
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Elements Had A Filled Outermost Shell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
