The Nucleus Of An Atom Contains
Juapaving
Mar 06, 2025 · 6 min read
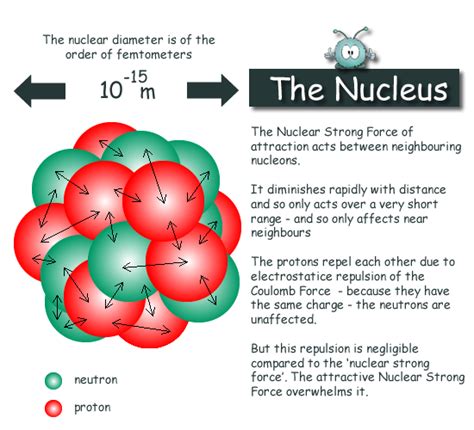
Table of Contents
The Nucleus of an Atom: A Deep Dive into its Composition and Significance
The atom, the fundamental building block of matter, is a fascinating realm of physics. While often depicted as a simple sphere, the atom possesses a complex internal structure. At its heart lies the nucleus, a tiny yet incredibly dense region that holds the key to an atom's identity and properties. This article delves deep into the composition of the atomic nucleus, exploring its constituent particles, its remarkable properties, and its crucial role in various scientific fields.
The Nucleus: A Dense Core of Protons and Neutrons
The nucleus, representing a minuscule fraction of the atom's overall volume, houses two types of subatomic particles: protons and neutrons. These particles, collectively known as nucleons, are bound together by a powerful force known as the strong nuclear force. This force, significantly stronger than the electromagnetic force repelling the positively charged protons, is responsible for the nucleus's stability.
Protons: The Positively Charged Identity Card
Protons carry a single positive electrical charge (+1) and possess a mass approximately 1836 times that of an electron. The number of protons in an atom's nucleus, known as its atomic number, defines the element. For instance, an atom with one proton is hydrogen, two protons is helium, and so on. This atomic number is fundamental to the element's chemical properties and its position on the periodic table.
Neutrons: The Neutral Stabilizers
Neutrons, as their name suggests, carry no electrical charge (0). Their mass is slightly larger than that of a proton. Neutrons play a crucial role in nuclear stability. In lighter elements, a roughly equal number of protons and neutrons is sufficient for stability. However, as the atomic number increases, the number of neutrons needed to maintain stability exceeds the number of protons. This excess of neutrons helps to counteract the repulsive forces between the positively charged protons. Without neutrons, the electromagnetic repulsion would overwhelm the strong nuclear force, causing the nucleus to disintegrate.
Isotopes: Variations on a Theme
Atoms of the same element can have different numbers of neutrons while retaining the same number of protons. These variations are called isotopes. Isotopes of an element have the same atomic number but different mass numbers, which represent the total number of protons and neutrons in the nucleus. For example, carbon-12 (⁶C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons.
Many isotopes are stable, meaning their nuclei remain intact over extended periods. However, some isotopes are radioactive, meaning their nuclei are unstable and undergo radioactive decay, emitting particles or energy to reach a more stable configuration. This radioactive decay is a crucial process in various scientific applications, including radiocarbon dating, medical imaging, and nuclear power generation.
Nuclear Forces: The Glue that Holds it Together
The stability of the atomic nucleus is a testament to the strength and complexity of the strong nuclear force. This force, unlike gravity and electromagnetism which operate over long distances, is a short-range force. It acts only over extremely short distances within the nucleus, effectively binding protons and neutrons together despite the electrostatic repulsion between protons.
The strong nuclear force is significantly stronger than the electromagnetic force, but its short range means that it only affects nucleons in close proximity. This characteristic explains why the nucleus is incredibly dense; the nucleons are packed tightly together due to the strong nuclear force's influence.
Another important force within the nucleus is the weak nuclear force. Although much weaker than the strong nuclear force, the weak force plays a vital role in radioactive decay, specifically beta decay, where a neutron transforms into a proton, emitting an electron and an antineutrino.
Nuclear Models: Understanding the Nucleus's Structure
Several models have been developed to understand the structure and behavior of the atomic nucleus. These models, while simplified representations, provide valuable insights into the complexities of nuclear physics.
The Liquid Drop Model
The liquid drop model likens the nucleus to a drop of liquid, where nucleons interact similarly to molecules in a liquid. This model explains several aspects of nuclear behavior, including nuclear fission, where a heavy nucleus splits into lighter nuclei.
The Shell Model
The shell model considers the nucleons to be arranged in energy levels or shells, similar to the arrangement of electrons in an atom. This model effectively explains the stability of certain nuclei with specific numbers of protons and neutrons, known as magic numbers. Nuclei with magic numbers of protons or neutrons exhibit enhanced stability.
The Collective Model
The collective model combines aspects of the liquid drop and shell models, considering both the collective motion of nucleons and their individual shell structure. This model provides a more comprehensive understanding of nuclear deformation and excitation.
The Nucleus and its Significance
The study of the atomic nucleus is not merely an academic pursuit; it has profound implications across numerous fields.
Nuclear Energy
The immense energy stored within the nucleus is harnessed in nuclear power plants. Nuclear fission, the splitting of heavy nuclei, releases enormous amounts of energy, which can be used to generate electricity.
Nuclear Medicine
Radioactive isotopes are used extensively in medical diagnosis and treatment. Techniques like PET (positron emission tomography) and SPECT (single-photon emission computed tomography) utilize radioactive tracers to image internal organs and detect diseases. Radiotherapy uses radioactive isotopes or radiation from accelerators to destroy cancerous cells.
Radiocarbon Dating
The radioactive decay of carbon-14 is used to date organic materials up to around 50,000 years old. This technique is crucial in archaeology, paleontology, and other fields studying ancient remains.
Material Science
Understanding nuclear reactions and interactions is vital in developing new materials with specific properties. Nuclear techniques are used to modify the properties of materials, making them stronger, more durable, or more resistant to corrosion.
Astrophysics
Nuclear processes play a crucial role in the life cycle of stars. Nuclear fusion, the combination of lighter nuclei into heavier ones, is the energy source of stars. Understanding nuclear reactions helps us comprehend stellar evolution and the formation of elements in the universe.
Conclusion: A Tiny Powerhouse
The atomic nucleus, though tiny and seemingly simple in its basic composition, is a complex and fascinating realm of physics. Its structure, governed by the strong and weak nuclear forces, determines the properties of atoms and elements. The study of the nucleus has led to remarkable advancements in various fields, from energy production to medical treatments and our understanding of the universe itself. Further research continues to unveil new secrets of this remarkable component of the atom, promising even greater breakthroughs in the future.
Latest Posts
Latest Posts
-
The Demand Curve Of A Monopolist Is
Mar 07, 2025
-
What Is The Lcm Of 7 And 12
Mar 07, 2025
-
What Is The Difference Between A Detritivore And A Decomposer
Mar 07, 2025
-
Common Factors Of 36 And 42
Mar 07, 2025
-
How Many Lines Of Symmetry Does A Regular Hexagon Have
Mar 07, 2025
Related Post
Thank you for visiting our website which covers about The Nucleus Of An Atom Contains . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
