The Diffusion Of Water Across A Selectively Permeable Membrane Is
Juapaving
Mar 16, 2025 · 6 min read
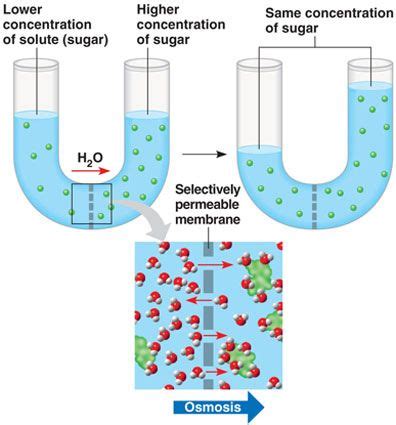
Table of Contents
The Diffusion of Water Across a Selectively Permeable Membrane: Osmosis Explained
The movement of water across a selectively permeable membrane is a fundamental process in biology, crucial for the survival and function of all living organisms. This process, known as osmosis, plays a vital role in various biological processes, from nutrient absorption in plants to maintaining cell turgor pressure and regulating blood volume in animals. Understanding osmosis requires a grasp of several key concepts, including selective permeability, concentration gradients, and the properties of water molecules. This comprehensive article will delve into the intricacies of osmosis, exploring its mechanisms, implications, and importance in various biological contexts.
Understanding Selective Permeability
Before delving into the specifics of osmosis, it's crucial to define selectively permeable membranes. These membranes, often composed of a phospholipid bilayer, act as gatekeepers, controlling the passage of substances into and out of cells or compartments. Their selectivity stems from the specific properties of the membrane's constituent molecules and the embedded proteins. Some substances can pass freely through the membrane (e.g., small, nonpolar molecules like oxygen and carbon dioxide), while others are selectively allowed passage (e.g., water, glucose, ions via channels or transporters) and still others are completely blocked (e.g., large polar molecules, charged particles without specific channels). This selective nature is critical for maintaining cellular homeostasis and the proper functioning of various biological systems.
The Role of Aquaporins
While water molecules are small and polar, their passage across cell membranes isn't solely determined by simple diffusion. Aquaporins, integral membrane proteins, facilitate the rapid movement of water across cell membranes. These channels selectively allow water molecules to pass through while excluding other molecules and ions, significantly increasing the rate of water transport compared to simple diffusion alone. The presence and abundance of aquaporins vary across different cell types and tissues, reflecting the varying demands for water transport in different physiological processes.
Osmosis: The Driving Force of Water Movement
Osmosis is the net movement of water molecules across a selectively permeable membrane from a region of higher water potential (or lower solute concentration) to a region of lower water potential (or higher solute concentration). This movement continues until equilibrium is reached, meaning the water potential is equal on both sides of the membrane. It's important to remember that osmosis is a passive process; it doesn't require energy input from the cell.
Water Potential: A Key Concept
Water potential is a measure of the free energy of water, representing the tendency of water to move from one area to another. It's influenced by several factors, including:
- Solute potential: The presence of dissolved solutes lowers the water potential. The more solutes present, the lower the water potential.
- Pressure potential: Pressure applied to the water increases its water potential (positive pressure potential), while tension reduces it (negative pressure potential). This is particularly relevant in plant cells with rigid cell walls.
- Matric potential: The attraction of water molecules to surfaces, such as cell walls or soil particles, also affects water potential.
Understanding the Direction of Water Movement
Water always moves from an area of higher water potential to an area of lower water potential. This means that:
- Water moves from a hypotonic solution (low solute concentration, high water potential) to a hypertonic solution (high solute concentration, low water potential).
- Water moves from a pure water solution (highest water potential) to a solution containing solutes (lower water potential).
Osmosis in Different Environments: Hypotonic, Hypertonic, and Isotonic Solutions
The behavior of cells in various environments is dictated by the osmotic relationship between the cell's internal environment and its surroundings.
-
Hypotonic Solution: In a hypotonic solution, the solute concentration outside the cell is lower than inside the cell. Water enters the cell, causing it to swell and potentially lyse (burst) in animal cells. In plant cells, the cell wall prevents lysis, resulting in turgor pressure, which is essential for plant support and growth.
-
Hypertonic Solution: In a hypertonic solution, the solute concentration outside the cell is higher than inside the cell. Water leaves the cell, causing it to shrink or plasmolyze. This is detrimental to both animal and plant cells. Plasmolysis in plants can lead to wilting and cell death.
-
Isotonic Solution: In an isotonic solution, the solute concentration inside and outside the cell is equal. There is no net movement of water, and the cell maintains its shape and volume.
The Importance of Osmosis in Biological Systems
Osmosis plays a critical role in numerous biological processes across various organisms:
1. Plant Physiology:
- Water Uptake: Osmosis is the primary mechanism by which plants absorb water from the soil through their roots. The water potential gradient between the soil and the root cells drives the movement of water into the plant.
- Turgor Pressure: The maintenance of turgor pressure, crucial for plant growth, support, and overall structural integrity, depends on osmosis. The inflow of water into plant cells creates turgor pressure, which pushes the cell membrane against the rigid cell wall.
- Stomatal Regulation: Osmosis plays a role in the regulation of stomatal opening and closing, which control gas exchange and water loss in plants.
2. Animal Physiology:
- Blood Volume Regulation: Osmosis is critical in maintaining blood volume and electrolyte balance. The kidneys play a crucial role in regulating water reabsorption in the nephrons, ensuring proper blood pressure and preventing dehydration or overhydration.
- Nutrient Absorption: Osmosis facilitates the absorption of nutrients and water from the digestive tract into the bloodstream.
- Cell Function: Osmosis maintains the proper hydration and osmotic balance of individual cells, ensuring their proper functioning and preventing damage.
3. Other Biological Processes:
- Water transport in xylem and phloem: Osmosis plays a key role in the long-distance transport of water and nutrients in plants through the vascular tissues, xylem and phloem.
- Maintaining cell shape: Osmotic pressure helps maintain cell structure and volume in various organisms.
Osmosis and Artificial Membranes: Applications in Technology
The principles of osmosis are not just confined to biological systems. They find applications in various technological advancements:
- Reverse Osmosis (RO): This technology uses pressure to overcome the osmotic gradient and force water across a semi-permeable membrane, separating it from impurities and solutes. RO is commonly used in water purification, desalination, and various industrial processes.
- Dialysis: Dialysis relies on osmosis and diffusion to remove waste products and excess fluids from the blood of individuals with kidney failure. An artificial semi-permeable membrane is used to filter the blood.
- Osmotic Power Generation: The osmotic pressure difference between freshwater and saltwater can be harnessed to generate electricity. This emerging technology offers a sustainable and environmentally friendly way to produce energy.
Conclusion
Osmosis, the diffusion of water across a selectively permeable membrane, is a fundamental process underlying many essential biological functions. From the absorption of water in plants to maintaining blood volume in animals, osmosis is a cornerstone of life. Understanding its mechanisms and implications is crucial for comprehending the complex interplay of water and solutes within living organisms. Furthermore, the application of osmosis principles in various technologies demonstrates its importance beyond biological systems, impacting diverse fields such as water purification, medical treatments, and energy generation. Continued research into osmosis promises to unravel further its complexities and lead to innovative advancements across multiple scientific disciplines.
Latest Posts
Latest Posts
-
What Is An Operator In Biology
Mar 17, 2025
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Diffusion Of Water Across A Selectively Permeable Membrane Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
