Strong Acid And Strong Base Titration
Juapaving
Mar 20, 2025 · 6 min read
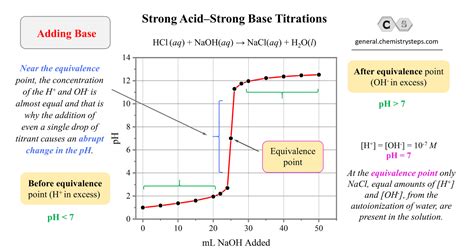
Table of Contents
Strong Acid-Strong Base Titration: A Comprehensive Guide
Strong acid-strong base titrations are a fundamental concept in chemistry, providing a crucial understanding of acid-base reactions and quantitative analysis. This detailed guide will explore the theoretical underpinnings, practical techniques, and applications of strong acid-strong base titrations, equipping you with a thorough grasp of this important topic.
Understanding the Basics: Acids, Bases, and Titration
Before delving into the specifics of strong acid-strong base titrations, let's establish a foundational understanding of the key terms involved.
What are Strong Acids and Strong Bases?
Strong acids are acids that completely dissociate in water, meaning they release all their hydrogen ions (H⁺) into the solution. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), nitric acid (HNO₃), and perchloric acid (HClO₄). Their complete dissociation leads to a high concentration of H⁺ ions, resulting in a low pH.
Strong bases are bases that completely dissociate in water, releasing all their hydroxide ions (OH⁻) into the solution. Common examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and lithium hydroxide (LiOH). The complete dissociation leads to a high concentration of OH⁻ ions, resulting in a high pH.
The Titration Process
Titration is a quantitative analytical technique used to determine the concentration of an unknown solution (the analyte) by reacting it with a solution of known concentration (the titrant). In a strong acid-strong base titration, a strong acid or a strong base is used as the titrant to neutralize the analyte. The reaction involves the combination of H⁺ ions from the acid and OH⁻ ions from the base to form water:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This reaction is highly favorable and proceeds essentially to completion.
The Titration Curve: A Visual Representation
The progress of a strong acid-strong base titration is best visualized through a titration curve. This curve plots the pH of the solution against the volume of titrant added. The shape of the curve provides valuable information about the titration, including the equivalence point.
Key Features of the Titration Curve:
-
Initial pH: The initial pH of the solution reflects the concentration of the strong acid or base being titrated. A strong acid will have a low initial pH, while a strong base will have a high initial pH.
-
Buffer Region (Minimal): Unlike weak acid-weak base titrations, strong acid-strong base titrations exhibit a minimal buffer region. This is because the complete dissociation of the strong acid or base prevents the formation of a significant buffer capacity.
-
Equivalence Point: The equivalence point is the point in the titration where the moles of acid and base are stoichiometrically equal. At this point, complete neutralization has occurred, and the pH is 7. This is a crucial point for determining the unknown concentration.
-
Sharp pH Change: The equivalence point is characterized by a dramatic and abrupt change in pH. This sharp change makes it relatively easy to identify the equivalence point visually or using a pH meter.
-
Post-Equivalence Point: After the equivalence point, the pH rapidly increases as excess titrant (base if titrating an acid, or acid if titrating a base) is added.
Calculating the Equivalence Point and Unknown Concentration
The equivalence point is crucial for determining the concentration of the unknown solution. This is achieved through stoichiometric calculations.
Stoichiometry and Calculations:
Consider the titration of a strong acid (HA) with a strong base (BOH). The balanced chemical equation is:
HA + BOH → BA + H₂O
The moles of acid can be calculated using:
Moles of HA = Molarity of HA × Volume of HA
Similarly, the moles of base are:
Moles of BOH = Molarity of BOH × Volume of BOH
At the equivalence point:
Moles of HA = Moles of BOH
Therefore, the unknown concentration can be calculated:
Molarity of HA = (Molarity of BOH × Volume of BOH) / Volume of HA
This calculation assumes a 1:1 stoichiometric ratio between the acid and base. For reactions with different stoichiometric ratios, adjustments must be made accordingly.
Indicators: Visualizing the Equivalence Point
While a pH meter offers precise measurements, indicators provide a visual method to detect the equivalence point. Indicators are weak acids or bases that change color depending on the pH of the solution.
Choosing the Right Indicator:
The choice of indicator depends on the pH change at the equivalence point. For strong acid-strong base titrations, the pH change is so sharp that a wide range of indicators will work effectively. Phenolphthalein is a commonly used indicator, changing from colorless to pink at a pH around 8.2. Other suitable indicators include methyl orange (pH range 3.1-4.4) and bromothymol blue (pH range 6.0-7.6).
Practical Considerations and Applications
Strong acid-strong base titrations are routinely used in various settings, demanding attention to detail in experimental procedures.
Experimental Setup:
Accurate measurements are paramount. Use clean, calibrated glassware (burettes, pipettes, volumetric flasks) to ensure precise volume measurements. A magnetic stirrer is helpful to ensure thorough mixing. A pH meter provides more precise results than indicators.
Error Analysis:
Potential sources of error include inaccurate measurements of volumes, impure solutions, and improper use of indicators or pH meters. Careful calibration and precise measurements are crucial to minimizing errors.
Applications:
Strong acid-strong base titrations find wide-ranging applications:
-
Determining the concentration of unknown acids or bases: This is the primary application, crucial in various analytical settings, from environmental monitoring to industrial quality control.
-
Acid-base equilibrium studies: The titration curves provide insights into the equilibrium constants and dissociation constants of acids and bases.
-
Food and beverage analysis: Titration is used to determine the acidity of food products like fruit juices or vinegar.
-
Pharmaceutical analysis: The purity and concentration of pharmaceutical drugs can be determined using titrations.
-
Environmental monitoring: Acid rain analysis and determining the pH of water samples utilize titration techniques.
-
Soil analysis: The acidity or alkalinity of soil can be determined through titration, providing valuable information for agriculture.
Advanced Concepts and Extensions
Beyond the basic principles, several advanced concepts expand the understanding of strong acid-strong base titrations:
Polyprotic Acids and Bases:
Titration of polyprotic acids (acids with more than one ionizable proton) or polyprotic bases results in multiple equivalence points on the titration curve, corresponding to the neutralization of each ionizable proton or hydroxide ion. The shape of the curve is more complex, reflecting the stepwise dissociation.
Non-Aqueous Titrations:
Some substances are not soluble or do not react appropriately in aqueous solutions. Non-aqueous titrations, using solvents other than water, are employed in such cases.
Potentiometric Titration:
Instead of using indicators, potentiometric titration uses a pH meter to monitor the pH change during the titration. This method offers greater accuracy and precision, particularly in situations where the pH change at the equivalence point is not very sharp.
Conclusion: A Powerful Analytical Tool
Strong acid-strong base titrations are a cornerstone of quantitative chemical analysis. Understanding the underlying principles, mastering the experimental techniques, and appreciating the various applications of this method empowers chemists and scientists to perform accurate and reliable analyses in a multitude of fields. By carefully controlling experimental variables and utilizing appropriate techniques, the precise determination of unknown concentrations is achievable, ensuring the reliability of results across diverse scientific disciplines. The simplicity of the underlying chemistry coupled with the wide applicability makes this technique an indispensable tool in the chemist's arsenal. From simple laboratory exercises to sophisticated industrial applications, strong acid-strong base titrations continue to play a vital role in scientific advancements and technological progress.
Latest Posts
Latest Posts
-
An Atom That Carries A Charge Is Called
Mar 21, 2025
-
95 Inches Is How Many Feet
Mar 21, 2025
-
Sodium Hydroxide And Hcl Balanced Equation
Mar 21, 2025
-
What Is The Lcm Of 8 And 11
Mar 21, 2025
-
Is A Foot Bigger Than A Yard
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Strong Acid And Strong Base Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
