Sodium Hydroxide And Hcl Balanced Equation
Juapaving
Mar 21, 2025 · 6 min read
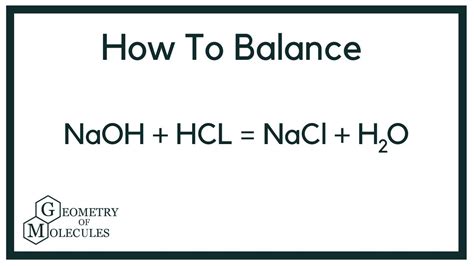
Table of Contents
Sodium Hydroxide and HCl: A Deep Dive into the Balanced Equation and its Implications
The reaction between sodium hydroxide (NaOH), a strong base, and hydrochloric acid (HCl), a strong acid, is a classic example of a neutralization reaction. Understanding this reaction, its balanced equation, and the implications it holds, is fundamental to chemistry and numerous applications across various fields. This comprehensive article will explore the reaction in detail, examining its stoichiometry, the resulting products, and its significance in different contexts.
The Balanced Chemical Equation
The reaction between sodium hydroxide and hydrochloric acid is a straightforward acid-base neutralization. The balanced chemical equation is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
This equation tells us that one mole of aqueous sodium hydroxide reacts with one mole of aqueous hydrochloric acid to produce one mole of aqueous sodium chloride and one mole of liquid water. The (aq) indicates that the substance is dissolved in water (aqueous solution), while (l) indicates it's in liquid form.
Understanding the Reaction Mechanism
At a molecular level, the reaction involves the transfer of a proton (H⁺) from the hydrochloric acid molecule to the hydroxide ion (OH⁻) of the sodium hydroxide molecule. The hydrogen ion, highly reactive, readily combines with the hydroxide ion to form a water molecule. The remaining sodium ion (Na⁺) and chloride ion (Cl⁻) remain in solution, forming an aqueous solution of sodium chloride. This is a highly exothermic reaction, meaning it releases heat.
Stoichiometry and Calculations
The balanced equation provides the stoichiometric ratios between reactants and products. This allows us to perform various calculations, such as determining the amount of product formed from a given amount of reactant, or the amount of reactant needed to completely neutralize a given amount of another reactant.
Example:
Let's say we have 10 grams of sodium hydroxide (NaOH) reacting with excess hydrochloric acid. How many grams of sodium chloride (NaCl) will be produced?
-
Calculate the moles of NaOH: The molar mass of NaOH is approximately 40 g/mol (23 g/mol for Na + 16 g/mol for O + 1 g/mol for H). Therefore, 10 g of NaOH is equal to 10 g / 40 g/mol = 0.25 moles of NaOH.
-
Use the mole ratio: According to the balanced equation, the mole ratio of NaOH to NaCl is 1:1. This means that 0.25 moles of NaOH will produce 0.25 moles of NaCl.
-
Calculate the mass of NaCl: The molar mass of NaCl is approximately 58.5 g/mol (23 g/mol for Na + 35.5 g/mol for Cl). Therefore, 0.25 moles of NaCl is equal to 0.25 moles * 58.5 g/mol = 14.625 g of NaCl.
Therefore, approximately 14.625 grams of sodium chloride will be produced. This calculation demonstrates the practical utility of the balanced equation in quantitative analysis.
Applications of the Reaction
The neutralization reaction between sodium hydroxide and hydrochloric acid has numerous applications across various fields, including:
1. Acid-Base Titrations
This reaction is crucial in acid-base titrations, a fundamental technique in analytical chemistry used to determine the concentration of an unknown acid or base solution. By carefully adding a standardized solution of NaOH (or HCl) to a solution of the unknown acid (or base), the point of neutralization (equivalence point) can be determined using an indicator. Knowing the volume and concentration of the standardized solution, the concentration of the unknown can be calculated.
2. Industrial Processes
In various industrial processes, controlling pH is critical. The reaction between NaOH and HCl is used to adjust the pH of solutions to the desired level. For example, in wastewater treatment, neutralizing acidic wastewater is essential before disposal. Similarly, in chemical manufacturing, maintaining precise pH levels is crucial for optimal reaction conditions and product quality.
3. Chemical Synthesis
The reaction can be used as a crucial step in certain chemical syntheses. The production of sodium chloride itself is a simple example, but more complex reactions may utilize the neutralization process as a means of generating a specific salt or altering the pH of a reaction mixture to favor a particular outcome.
4. Digestive System
Although not directly a practical application in the same way as industrial processes, understanding this reaction is essential for comprehending the workings of the human body. The stomach produces hydrochloric acid to aid digestion. If excessive stomach acid is present (hyperacidity), antacids containing bases like sodium hydroxide (although rarely used directly in this form due to its corrosiveness) can neutralize the excess acid, relieving discomfort.
Safety Precautions
Both sodium hydroxide and hydrochloric acid are corrosive chemicals. They can cause severe burns to the skin and eyes. Therefore, handling these substances requires utmost caution and adherence to safety protocols:
- Always wear appropriate personal protective equipment (PPE): This includes safety glasses, gloves, and lab coats.
- Perform the reaction in a well-ventilated area: The reaction can produce heat, and the fumes can be irritating.
- Add the acid to the base slowly: This helps to control the rate of the exothermic reaction and minimize splashing.
- Neutralize any spills immediately: Use appropriate neutralizing agents and follow the established safety procedures for chemical spills.
Beyond the Basics: Exploring Related Concepts
The simple neutralization reaction between NaOH and HCl provides a strong foundation for understanding more complex chemical concepts.
1. Strong vs. Weak Acids and Bases:
While HCl and NaOH are strong acids and bases, respectively (they completely dissociate in water), many other acids and bases are weak. Weak acids and bases only partially dissociate in water, leading to more complex equilibrium considerations and different reaction kinetics. The resulting pH changes would also be different.
2. Titration Curves:
Graphically representing the pH change during a titration provides valuable insights into the acid-base reaction. Titration curves show the equivalence point and help determine the pKa or pKb of weak acids and bases. The shape of the curve reflects the strength of the acid and base involved.
3. Salt Hydrolysis:
Although NaCl is a neutral salt (formed from a strong acid and strong base), salts formed from weak acids or bases can undergo hydrolysis, affecting the pH of the resulting solution. Understanding hydrolysis is crucial for predicting the pH of salt solutions.
4. Buffer Solutions:
Buffer solutions resist changes in pH upon addition of small amounts of acid or base. These solutions are often prepared using a weak acid and its conjugate base, or a weak base and its conjugate acid. Understanding neutralization reactions is essential for understanding how buffer solutions function.
Conclusion
The reaction between sodium hydroxide and hydrochloric acid, represented by the balanced equation NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l), is a fundamental chemical reaction with widespread applications. From analytical chemistry techniques like titrations to industrial processes requiring pH control, this reaction plays a vital role. Understanding the stoichiometry, safety precautions, and related concepts such as strong vs. weak acids and bases, titration curves, salt hydrolysis, and buffer solutions provides a deep understanding of this essential chemical process. Remembering the importance of safety when handling corrosive chemicals is paramount. Always prioritize safe laboratory practices when working with acids and bases.
Latest Posts
Latest Posts
-
Where Is Most Of The Atp Made During Cellular Respiration
Mar 28, 2025
-
Calculate Water Pressure From Tank Height
Mar 28, 2025
-
What Numbers Can Go Into 27
Mar 28, 2025
-
Write 66 As A Product Of Prime Factors
Mar 28, 2025
-
Points That Lie On The Same Line Are Collinear
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Sodium Hydroxide And Hcl Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
