Smallest Particle Of An Element That Retains Its Properties
Juapaving
Mar 09, 2025 · 7 min read
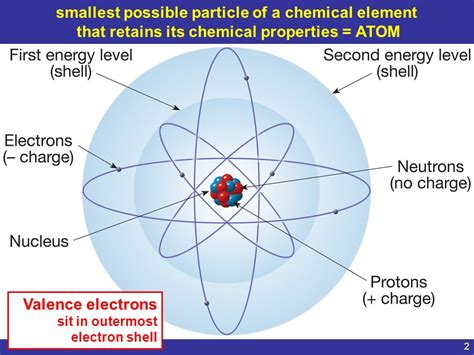
Table of Contents
The Atom: The Smallest Particle Retaining Elemental Properties
The quest to understand the fundamental building blocks of matter has captivated humanity for millennia. From ancient Greek philosophers pondering the nature of reality to modern-day physicists exploring the intricacies of quantum mechanics, the pursuit of knowledge at the atomic level has driven scientific advancement. At the heart of this quest lies a crucial concept: the atom, the smallest particle of an element that retains its chemical properties. This article delves deep into the fascinating world of atoms, exploring their structure, behavior, and significance in shaping our understanding of the universe.
Defining the Atom: A Building Block of Matter
The word "atom" originates from the Greek word "atomos," meaning "indivisible." While early thinkers conceived of atoms as indivisible spheres, modern science reveals a far more intricate reality. An atom is the smallest unit of an element that maintains the chemical properties of that element. This means that breaking an atom down further will result in particles (like protons, neutrons, and electrons) that no longer exhibit the characteristics of the original element. Instead, they possess properties unique to their subatomic nature.
Think of it like this: imagine a LEGO castle. The individual LEGO bricks are like atoms – each brick retains its unique shape and properties. You can assemble them to create a complex structure, but breaking a single brick down won't give you a smaller brick that still looks and acts like a LEGO brick. Similarly, breaking down an atom of oxygen doesn't yield a smaller unit that still acts like oxygen.
The Structure of an Atom: A Subatomic World
Despite being incredibly small, atoms possess an intricate internal structure. They are composed primarily of three types of subatomic particles:
1. Protons: Positively Charged Core
Located within the atom's nucleus, protons carry a positive electrical charge. The number of protons in an atom's nucleus determines the element's atomic number, which uniquely identifies it on the periodic table. For example, all hydrogen atoms have one proton, all helium atoms have two, and so on. The atomic number is a fundamental property that dictates an element's chemical behavior.
2. Neutrons: Neutral Nuclear Companions
Also residing in the atom's nucleus, neutrons carry no electrical charge—they are electrically neutral. They contribute to the atom's mass but not its charge. The number of neutrons in an atom's nucleus can vary, giving rise to different isotopes of the same element. Isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. For example, carbon-12 and carbon-14 are both isotopes of carbon, differing only in their neutron count.
3. Electrons: Orbiting Negatively Charged Particles
Unlike protons and neutrons, electrons are located outside the nucleus in regions called electron shells or orbitals. They carry a negative electrical charge, equal in magnitude but opposite in sign to the charge of a proton. The number of electrons in an atom is typically equal to the number of protons, making the atom electrically neutral. However, atoms can gain or lose electrons, forming ions, which are charged particles. The arrangement of electrons in an atom's shells determines its chemical reactivity and how it interacts with other atoms.
Atomic Mass and Isotopes: Variations Within an Element
The atomic mass of an atom is determined by the total number of protons and neutrons in its nucleus. Since isotopes have varying numbers of neutrons, they also have different atomic masses. The atomic mass listed on the periodic table is a weighted average of the atomic masses of all the naturally occurring isotopes of an element, accounting for their relative abundance. This weighted average is also known as the relative atomic mass or atomic weight.
Understanding isotopes is crucial in various fields, including:
-
Radioactive Dating: Certain isotopes are radioactive, meaning they undergo spontaneous decay, emitting radiation. This property is used to determine the age of rocks, fossils, and other materials in a technique called radiocarbon dating.
-
Medical Imaging: Radioactive isotopes are also used in medical imaging techniques, like PET (positron emission tomography) scans, allowing doctors to visualize internal organs and detect diseases.
-
Nuclear Medicine: Isotopes are employed in nuclear medicine for treating certain types of cancer.
Chemical Bonding: Atoms Interacting
Atoms rarely exist in isolation. They interact with other atoms to form molecules and compounds, driven by the desire to achieve a stable electron configuration, often a full outer electron shell. This interaction occurs through different types of chemical bonds:
1. Ionic Bonds: Electron Transfer
Ionic bonds form when one atom transfers one or more electrons to another atom. This creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions holds them together in an ionic compound. Sodium chloride (table salt) is a classic example of an ionic compound.
2. Covalent Bonds: Electron Sharing
Covalent bonds form when atoms share electrons to achieve a stable electron configuration. This sharing creates a strong bond between the atoms, forming a molecule. Water (H₂O) is a common example of a molecule held together by covalent bonds.
3. Metallic Bonds: Electron Sea
Metallic bonds are found in metals. In metallic bonding, the valence electrons are delocalized, meaning they are not associated with any particular atom but rather form a "sea" of electrons that surround the positively charged metal ions. This "sea" of electrons is responsible for the characteristic properties of metals, such as high electrical and thermal conductivity, malleability, and ductility.
The Periodic Table: Organizing the Elements
The periodic table is a powerful tool that organizes all known elements based on their atomic number, electron configuration, and recurring chemical properties. Elements are arranged in periods (rows) and groups (columns), reflecting the patterns in their electron configurations. Elements within the same group exhibit similar chemical properties due to the similar arrangement of their valence electrons. The periodic table provides a wealth of information about the elements, including their atomic mass, electronegativity, and reactivity.
Beyond the Atom: Subatomic Particles and Quantum Mechanics
While the atom is the smallest particle retaining elemental properties, it's crucial to understand that it's not indivisible. The subatomic particles – protons, neutrons, and electrons – themselves have a rich internal structure and behavior governed by the principles of quantum mechanics.
Protons and neutrons are composed of even smaller particles called quarks, which are held together by the strong nuclear force. Electrons, on the other hand, are considered fundamental particles, meaning they are not composed of smaller constituents. The behavior of these subatomic particles is described by quantum mechanics, which dictates that particles also exhibit wave-like properties, challenging classical notions of physics.
The Significance of Atoms in Science and Technology
The understanding of atoms and their behavior has revolutionized countless fields, including:
-
Chemistry: Chemistry fundamentally relies on the study of atoms and their interactions, allowing us to understand chemical reactions, synthesize new materials, and develop new technologies.
-
Physics: Atomic physics and nuclear physics explore the structure and behavior of atoms and their nuclei, leading to breakthroughs in energy production, medical imaging, and materials science.
-
Biology: Atoms are the fundamental building blocks of all living organisms. Understanding atomic structure and interactions is essential to comprehending biological processes, such as metabolism and DNA replication.
-
Material Science: The properties of materials are directly related to the arrangement and interactions of their constituent atoms. This understanding allows scientists and engineers to design and synthesize materials with specific properties for various applications.
-
Medicine: Radioactive isotopes and atomic-level understanding of biological processes are crucial in diagnosis and treatment of diseases. Medical imaging and radiation therapy are prime examples.
-
Technology: The development of modern technologies, such as transistors and integrated circuits, is fundamentally based on our understanding of atomic and electronic properties. These advancements have revolutionized computing, communication, and countless other aspects of modern life.
Conclusion: The Enduring Mystery and Importance of the Atom
The atom, the smallest particle of an element retaining its properties, remains a subject of intense scientific curiosity and study. While we have made significant strides in understanding its structure and behavior, there are still many unanswered questions, particularly at the quantum level. The exploration of the atom and its subatomic constituents continues to drive innovation and discovery, shaping our understanding of the universe and pushing the boundaries of human knowledge and technological advancement. The seemingly simple concept of the atom underpins the complexity of the world around us, highlighting its profound significance in science, technology, and our daily lives. Its enduring mystery and importance guarantee its continued study for generations to come.
Latest Posts
Latest Posts
-
Five Letter Words End In Er
Mar 09, 2025
-
What Mountain Range Separates Europe And Asia
Mar 09, 2025
-
What Is The Difference Between A Duty And A Responsibility
Mar 09, 2025
-
Which Best Describes The Law Of Conservation Of Mass
Mar 09, 2025
-
1 Out Of 6 Is What Percentage
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about Smallest Particle Of An Element That Retains Its Properties . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
