Which Best Describes The Law Of Conservation Of Mass
Juapaving
Mar 09, 2025 · 6 min read
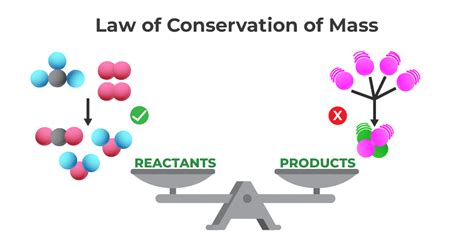
Table of Contents
Which Best Describes the Law of Conservation of Mass? A Deep Dive
The Law of Conservation of Mass, a cornerstone of chemistry and physics, declares that matter cannot be created or destroyed in a chemical reaction. While seemingly simple, this fundamental principle has profound implications across numerous scientific fields, shaping our understanding of everything from chemical reactions to nuclear processes. This comprehensive article explores the law in detail, examining its nuances, applications, and exceptions, providing a thorough understanding for students and enthusiasts alike.
Understanding the Core Principle: Matter Remains Constant
At its heart, the Law of Conservation of Mass asserts that the total mass of reactants in a chemical reaction equals the total mass of the products. This means that during a chemical transformation, atoms are neither gained nor lost; they simply rearrange themselves to form new substances. Consider a simple reaction, the burning of wood. While the wood visibly disappears, transforming into ash and smoke, the total mass remains constant. The mass of the wood, oxygen consumed during combustion, and any other reactants equals the combined mass of ash, smoke, and any other products.
Key Concepts to Grasp
-
Mass: This refers to the amount of matter in an object. It's crucial to differentiate mass from weight, which is the force exerted on an object due to gravity. Mass remains consistent regardless of location, while weight varies.
-
Chemical Reaction: A process where substances undergo a chemical change, resulting in the formation of new substances with different properties. Chemical reactions involve the breaking and formation of chemical bonds.
-
Reactants: The starting materials in a chemical reaction.
-
Products: The substances formed as a result of a chemical reaction.
-
Closed System: The law of conservation of mass strictly applies to closed systems, where no matter can enter or leave. Open systems, where matter exchange occurs, can show apparent mass changes.
Historical Context: Lavoisier's Contributions
Antoine Lavoisier, a renowned 18th-century French chemist often called the "father of modern chemistry," is credited with formulating the Law of Conservation of Mass. Through meticulous experimentation, particularly his work on combustion, Lavoisier demonstrated that the total mass of the reactants always equaled the total mass of the products in a closed system. His experiments involved carefully weighing reactants and products, eliminating any loss or gain of mass during reactions. This rigorous approach established the law as a fundamental principle.
Lavoisier's Experiments and Their Significance
Lavoisier's experiments were not just about weighing substances; they were about systematically controlling variables. He designed experiments to minimize sources of error, ensuring accurate mass measurements. His work showcased the importance of careful observation and quantitative analysis in scientific inquiry, paving the way for future advancements in chemistry.
Applications Across Scientific Disciplines
The Law of Conservation of Mass has far-reaching implications in numerous fields, including:
-
Stoichiometry: This branch of chemistry relies heavily on the law to calculate the amounts of reactants and products in chemical reactions. By understanding the mass relationships, chemists can precisely predict the yields of reactions.
-
Environmental Science: Understanding the conservation of mass is vital for analyzing environmental processes, such as pollution dispersal and nutrient cycling in ecosystems. Tracking the movement of pollutants requires applying the principle of mass balance.
-
Industrial Chemistry: Industrial processes rely on precise mass balances to optimize production and minimize waste. Chemical engineers utilize the law to design efficient and effective chemical manufacturing processes.
-
Nuclear Chemistry: While seemingly an exception, the Law of Conservation of Mass still holds true even in nuclear reactions. The small apparent loss of mass in nuclear reactions is converted into an equivalent amount of energy, as described by Einstein's famous equation, E=mc².
Addressing Apparent Exceptions and Limitations
While the Law of Conservation of Mass is highly accurate in most chemical reactions, there are seemingly apparent exceptions that require further examination:
-
Open Systems: As previously mentioned, in open systems, where matter can be exchanged with the surroundings, the law doesn't directly apply. Mass may appear to be gained or lost due to the entry or exit of substances. For instance, a plant growing appears to gain mass, but this is due to the absorption of CO2 and water.
-
Nuclear Reactions: Nuclear reactions involve transformations at the atomic nucleus level, where a small amount of mass is converted into a tremendous amount of energy. Einstein's mass-energy equivalence principle explains this apparent mass discrepancy. However, the total mass-energy remains conserved.
-
High-Energy Physics: At the subatomic level, in particle physics experiments involving extremely high energies, the creation and annihilation of particles occur. While mass may seemingly be created or destroyed, the total energy remains conserved, again reaffirming the broader principle of mass-energy conservation.
The Broader Context: Mass-Energy Equivalence
Einstein's famous equation, E=mc², elegantly connects mass and energy. It indicates that mass and energy are interchangeable and that a small amount of mass can be converted into a large amount of energy, and vice versa. This is crucial in understanding nuclear reactions where a small loss in mass results in a significant release of energy. Therefore, while the Law of Conservation of Mass is a simplification, the broader principle of conservation of mass-energy holds true across all physical processes.
Reconciling Apparent Discrepancies
The apparent exceptions to the Law of Conservation of Mass are not true violations but rather instances where the broader principle of mass-energy conservation applies. The conversion of mass to energy, particularly evident in nuclear reactions, highlights the interconnectedness of mass and energy and reinforces the fundamental principle of conservation.
Practical Applications and Everyday Examples
The Law of Conservation of Mass is not confined to laboratory experiments; it impacts our daily lives in countless ways:
-
Cooking: When you cook, the total mass of ingredients used should theoretically equal the total mass of the final dish. Any apparent loss is due to evaporation of water or other volatile compounds.
-
Recycling: Recycling relies on the principle that materials are not destroyed but can be transformed into new products. Recycling conserves resources and minimizes waste.
-
Combustion Engines: In a car engine, the burning of fuel involves a chemical reaction where the mass of the fuel and oxygen equals the mass of the exhaust gases and energy released.
-
Environmental Impact Assessments: Environmental agencies use mass balance calculations to assess the impact of industrial processes on ecosystems and to track pollutants' movement and transformation.
Conclusion: A Fundamental Principle Enduring Through Time
The Law of Conservation of Mass, though refined by our understanding of mass-energy equivalence, remains a cornerstone principle in science. Its implications are vast, spanning across chemistry, physics, environmental science, and various engineering disciplines. Understanding this principle provides a crucial foundation for comprehending chemical reactions, analyzing environmental processes, and designing efficient industrial procedures. While apparent exceptions exist, the broader principle of conservation of mass-energy ensures that matter and energy, while interconvertible, are always conserved in the universe. This timeless law continues to shape our scientific understanding and technological advancements.
Latest Posts
Latest Posts
-
How Many Milliseconds Are In A Minute
Mar 10, 2025
-
How Many Valence Electrons Does Copper Have
Mar 10, 2025
-
How Many Mm In 1 M
Mar 10, 2025
-
How Many Feet Is 118 Inches
Mar 10, 2025
-
Whats The Square Root Of 400
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Which Best Describes The Law Of Conservation Of Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
