Relationship Between Density Mass And Volume
Juapaving
Mar 16, 2025 · 7 min read
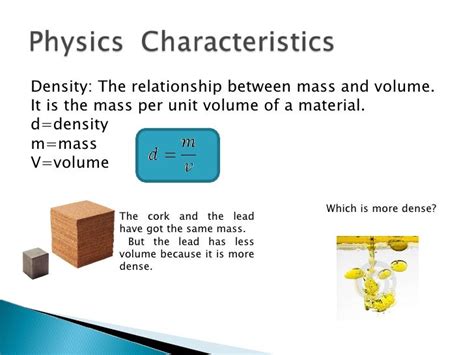
Table of Contents
The Intimate Relationship Between Density, Mass, and Volume
Understanding the relationship between density, mass, and volume is fundamental to numerous scientific fields, from physics and chemistry to engineering and geology. This seemingly simple relationship underpins our comprehension of how matter behaves and interacts within the universe. This comprehensive guide will delve deep into this interconnected trio, exploring their definitions, the mathematical relationship binding them, and real-world applications showcasing their importance.
Defining the Key Players: Density, Mass, and Volume
Before we dive into the intricate dance between these three properties, let's clearly define each term:
Mass: The Measure of Inertia
Mass is a scalar quantity representing the amount of matter in an object. It dictates an object's resistance to acceleration (inertia). A larger mass requires a greater force to achieve the same acceleration as a smaller mass. Mass is typically measured in kilograms (kg) in the International System of Units (SI). It's crucial to distinguish mass from weight; weight is the force exerted on an object due to gravity, while mass is an intrinsic property independent of gravitational forces. For instance, an astronaut's mass remains constant on the moon, even though their weight is significantly less due to the weaker lunar gravity.
Volume: The Measure of Space Occupied
Volume measures the amount of three-dimensional space occupied by an object or substance. This can refer to the space enclosed within a container or the space occupied by a solid object. Volume is typically measured in cubic meters (m³) in the SI system, although other units like liters (L) and cubic centimeters (cm³) are also commonly used. The choice of unit depends on the scale of the object being measured. For example, the volume of a swimming pool is typically measured in cubic meters, while the volume of a drop of water is measured in cubic centimeters or milliliters.
Density: Mass per Unit Volume
Density is a crucial physical property defined as the mass per unit volume of a substance. It essentially tells us how tightly packed the matter is within a given space. A high-density substance means a large amount of mass is packed into a small volume, while a low-density substance has a smaller amount of mass spread over a larger volume. Density is typically denoted by the Greek letter ρ (rho) and is calculated by dividing mass (m) by volume (V):
ρ = m/V
This simple equation forms the bedrock of understanding the relationship between these three properties. Knowing any two of these values allows us to calculate the third.
The Interplay: Calculating Density, Mass, and Volume
The formula ρ = m/V allows for straightforward calculations:
Calculating Density
To calculate the density of an object, you need to know its mass and volume. For example, if an object has a mass of 10 kg and a volume of 2 m³, its density would be:
ρ = 10 kg / 2 m³ = 5 kg/m³
Calculating Mass
If you know the density and volume of an object, you can calculate its mass by rearranging the formula:
m = ρ * V
For instance, if a substance has a density of 8 g/cm³ and a volume of 5 cm³, its mass would be:
m = 8 g/cm³ * 5 cm³ = 40 g
Calculating Volume
Similarly, if you know the density and mass of an object, you can calculate its volume:
V = m / ρ
For example, if an object has a mass of 20 kg and a density of 4 kg/m³, its volume would be:
V = 20 kg / 4 kg/m³ = 5 m³
Density: A Distinguishing Property of Matter
Density is a characteristic property of a substance, meaning it's constant for a given substance under specific conditions (temperature and pressure). This property allows us to identify and distinguish between different materials. For instance, gold has a much higher density than wood, meaning a small volume of gold will have a significantly greater mass than the same volume of wood. This difference in density is exploited in various applications, such as gold prospecting, where the higher density of gold allows it to be separated from lighter materials using methods like panning.
Factors Affecting Density
While density is a characteristic property, it's not entirely immutable. Several factors can influence the density of a substance:
-
Temperature: Generally, increasing the temperature of a substance causes it to expand, increasing its volume while keeping its mass constant. This leads to a decrease in density. Water is a notable exception, exhibiting an anomalous expansion behavior near its freezing point.
-
Pressure: Increasing pressure on a substance compresses it, decreasing its volume and increasing its density. This effect is more pronounced in gases than in solids or liquids.
-
Phase Changes: The density of a substance changes dramatically during phase transitions (solid, liquid, gas). Ice, for instance, is less dense than liquid water, which is why ice floats.
Real-World Applications of Density, Mass, and Volume
The concepts of density, mass, and volume are ubiquitous in various aspects of our lives and scientific endeavors:
Archimedes' Principle and Buoyancy
Archimedes' principle, which states that an object immersed in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced, relies heavily on the concept of density. An object will float if its average density is less than the density of the fluid it's immersed in, and it will sink if its density is greater. This principle underpins the design of ships, submarines, and even hot air balloons.
Material Science and Engineering
Engineers and material scientists utilize density calculations extensively in designing structures and selecting materials. Knowing the density of a material allows for accurate estimations of the weight and structural integrity of a component. This is vital in areas like aerospace engineering, where weight is a critical factor.
Geology and Geophysics
Density plays a crucial role in understanding the Earth's internal structure. Seismic waves travel at different speeds through materials of varying densities, providing valuable insights into the composition and structure of the Earth's layers. Density measurements are also vital in mineral exploration and resource assessment.
Meteorology and Oceanography
Density differences in air masses drive weather patterns. Warmer, less dense air rises, while cooler, denser air sinks, creating pressure gradients that generate wind. Similarly, density variations in ocean water influence ocean currents and marine ecosystems.
Advanced Concepts and Further Exploration
The relationship between density, mass, and volume extends to more complex scenarios:
Density of Mixtures and Solutions
Calculating the density of mixtures or solutions requires considering the densities and proportions of the individual components. This is often crucial in chemistry and chemical engineering, where the density of a solution dictates its properties and behavior.
Density and Pressure in Gases: Ideal Gas Law
The density of gases is particularly sensitive to pressure and temperature. The ideal gas law provides a framework for understanding and calculating the density of gases under various conditions. This is essential in various applications, including atmospheric science and industrial gas processing.
Relative Density (Specific Gravity)
Relative density, also known as specific gravity, is the ratio of the density of a substance to the density of a reference substance, typically water. This dimensionless quantity is often used to compare the densities of different materials.
Conclusion: A Foundation for Understanding the World
The seemingly simple relationship between density, mass, and volume forms the cornerstone of understanding how matter behaves and interacts. This fundamental concept underpins countless applications across numerous scientific and engineering disciplines. From predicting the buoyancy of a ship to understanding the Earth's internal structure, the power of this interconnected trio is undeniable. By mastering these concepts, we gain a deeper appreciation for the intricate workings of the physical world around us. Further exploration into the nuances of density, mass, and volume will continue to yield valuable insights and advancements in various fields of study.
Latest Posts
Latest Posts
-
What Is The Formula For The Compound Iron Iii Sulfate
Mar 17, 2025
-
What Is 3 6 As A Percent
Mar 17, 2025
-
How Many Chambers Does A Frogs Heart Have
Mar 17, 2025
-
How Many Feet Is 300 M
Mar 17, 2025
-
Sulfur Number Of Protons Neutrons And Electrons
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Relationship Between Density Mass And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
