Reaction Of Iron And Hydrochloric Acid
Juapaving
Mar 18, 2025 · 6 min read
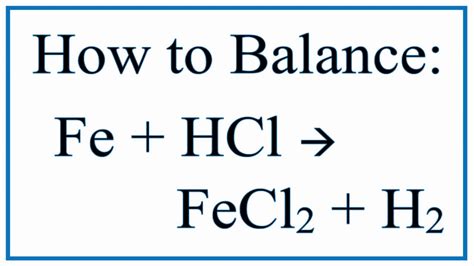
Table of Contents
The Reaction of Iron and Hydrochloric Acid: A Deep Dive
The reaction between iron (Fe) and hydrochloric acid (HCl) is a classic example of a single displacement reaction, a fundamental concept in chemistry. Understanding this reaction provides a foundational understanding of redox reactions, stoichiometry, and the properties of both metals and acids. This comprehensive guide will delve into the intricacies of this reaction, exploring its chemical equation, the factors influencing its rate, safety precautions, and practical applications.
The Chemical Reaction: Understanding the Fundamentals
The reaction between iron and hydrochloric acid is an exothermic reaction, meaning it releases heat. Iron, being a relatively reactive metal, readily displaces the hydrogen ions (H⁺) from the hydrochloric acid, forming iron(II) chloride (FeCl₂) and hydrogen gas (H₂).
The balanced chemical equation is:
Fe(s) + 2HCl(aq) → FeCl₂(aq) + H₂(g)
This equation shows that one mole of solid iron reacts with two moles of aqueous hydrochloric acid to produce one mole of aqueous iron(II) chloride and one mole of hydrogen gas. The "(s)", "(aq)", and "(g)" denote the physical states of the substances: solid, aqueous (dissolved in water), and gas, respectively.
Oxidation and Reduction: A Redox Perspective
This reaction is also a redox (reduction-oxidation) reaction. Iron undergoes oxidation, losing two electrons to form the Fe²⁺ ion:
Fe(s) → Fe²⁺(aq) + 2e⁻
Simultaneously, the hydrogen ions in the hydrochloric acid undergo reduction, gaining electrons to form hydrogen gas:
2H⁺(aq) + 2e⁻ → H₂(g)
The transfer of electrons from iron to hydrogen ions is the driving force behind the reaction. The iron acts as the reducing agent (donating electrons), while the hydrogen ions act as the oxidizing agent (accepting electrons).
Factors Affecting the Reaction Rate
Several factors influence the rate at which iron reacts with hydrochloric acid. Understanding these factors is crucial for controlling the reaction and predicting its outcome.
Concentration of Hydrochloric Acid
A higher concentration of hydrochloric acid leads to a faster reaction rate. This is because a higher concentration means a greater number of hydrogen ions are available to react with the iron. More collisions occur per unit time, increasing the likelihood of successful reactions.
Surface Area of Iron
Increasing the surface area of the iron increases the reaction rate. A finely divided iron powder will react much faster than a single, large piece of iron. This is because a larger surface area provides more sites for the acid to react with. Think of it like trying to dissolve a sugar cube versus a spoonful of granulated sugar – the granulated sugar dissolves faster due to its increased surface area.
Temperature
Increasing the temperature accelerates the reaction rate. Higher temperatures provide the reacting molecules with greater kinetic energy, leading to more frequent and energetic collisions. This increased energy overcomes the activation energy barrier, allowing more reactions to occur successfully.
Presence of Impurities
The presence of impurities on the surface of the iron can either inhibit or catalyze the reaction. Impurities might form a protective layer, hindering the access of the acid to the iron, slowing the reaction down. Conversely, some impurities might act as catalysts, speeding up the reaction.
Safety Precautions: Handling with Care
The reaction between iron and hydrochloric acid generates hydrogen gas, which is highly flammable. Therefore, it is crucial to take appropriate safety precautions when performing this experiment.
- Always perform the reaction in a well-ventilated area or under a fume hood. Hydrogen gas is lighter than air and can accumulate in enclosed spaces, posing a fire hazard.
- Avoid open flames or sparks near the reaction vessel. Any ignition source could ignite the hydrogen gas, leading to an explosion.
- Wear appropriate safety goggles and gloves. Hydrochloric acid is corrosive, and accidental splashes can cause serious injury.
- Dispose of the reaction mixture properly. Follow your institution's guidelines for the disposal of chemical waste.
Observing the Reaction: Visual and Measurable Changes
The reaction between iron and hydrochloric acid is readily observable through several visual and measurable changes:
- Effervescence: The most noticeable observation is the production of hydrogen gas, which manifests as bubbles forming and rising to the surface of the liquid.
- Dissolution of Iron: The iron metal gradually dissolves as it reacts with the acid.
- Color Change: The solution's color might change slightly as iron(II) chloride forms. The exact color change can depend on the concentration of the reactants and the presence of impurities.
- Temperature Increase: The reaction is exothermic, meaning it releases heat. You can detect a temperature increase using a thermometer.
Applications of the Reaction
The reaction between iron and hydrochloric acid, while seemingly simple, has several important applications:
- Production of Iron(II) Chloride: Iron(II) chloride is a valuable chemical compound with numerous applications in various industries, including water treatment, photography, and as a mordant in dyeing. This reaction provides a straightforward method for its production.
- Cleaning Metal Surfaces: The reaction can be used to remove rust (iron oxide) from metal surfaces. The acid reacts with the iron oxide, dissolving it and leaving a clean metal surface behind.
- Testing for the Presence of Iron: The reaction can be used as a qualitative test for the presence of iron in a sample. The production of hydrogen gas and the dissolution of the metal are indicative of an iron-containing sample reacting with hydrochloric acid.
- Educational Purposes: This reaction is a cornerstone of many introductory chemistry courses. It illustrates fundamental concepts such as redox reactions, stoichiometry, and the reactivity of metals.
Beyond Iron(II) Chloride: Exploring Other Possibilities
While the formation of iron(II) chloride is the most common outcome, the reaction's products can vary depending on conditions. For instance, in the presence of oxygen, iron(III) chloride (FeCl₃) might also form. The presence of oxidizing agents can further influence the oxidation state of the iron.
Understanding the reaction conditions is crucial in controlling the outcome. Factors like the concentration of acid, temperature, presence of oxygen, and the purity of the iron all play significant roles.
Further Investigation and Experimentation
This reaction provides a rich foundation for further investigation. Students and enthusiasts can perform experiments to quantitatively determine the reaction rate under different conditions, exploring the effects of concentration, temperature, and surface area. They can also investigate the stoichiometry of the reaction, verifying the balanced chemical equation through careful measurements.
Conclusion: A Reaction with Broad Significance
The seemingly simple reaction between iron and hydrochloric acid is a window into a world of chemical complexities. From the fundamental principles of redox reactions to its various practical applications, this reaction offers a wealth of educational and practical value. Understanding this reaction provides a crucial stepping stone for deeper explorations in chemistry and its applications in various fields. Through careful observation, experimentation, and an understanding of the underlying chemical principles, we can unlock a deeper appreciation for this foundational reaction. Remember always to prioritize safety when working with acids and reactive metals.
Latest Posts
Latest Posts
-
32 Degrees C Is What In Fahrenheit
Mar 19, 2025
-
Which Of The Following Is Not Found Within Dna
Mar 19, 2025
-
A Generator Is A Device That Converts Energy Into Energy
Mar 19, 2025
-
What Is The Conservation Of Charge
Mar 19, 2025
-
How Many Faces On A Dice
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Reaction Of Iron And Hydrochloric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
