What Is The Conservation Of Charge
Juapaving
Mar 19, 2025 · 6 min read
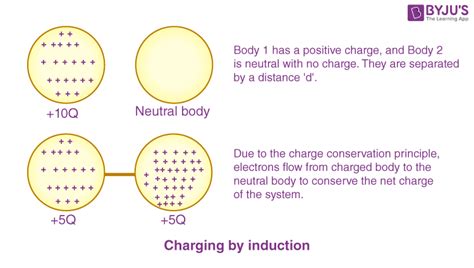
Table of Contents
What is the Conservation of Charge? A Deep Dive into a Fundamental Law of Physics
The conservation of charge is a fundamental principle in physics, stating that the total electric charge in an isolated system remains constant over time. This means that charge can neither be created nor destroyed, only transferred from one object to another. It's a cornerstone of our understanding of electromagnetism and underpins numerous phenomena in the universe, from the behavior of subatomic particles to the workings of electronic circuits. This article will delve into the intricacies of charge conservation, exploring its implications, applications, and the experimental evidence that supports this crucial law.
Understanding Electric Charge
Before delving into the conservation of charge, let's establish a firm understanding of electric charge itself. Electric charge is a fundamental property of matter, much like mass. Objects possess charge due to the presence of elementary particles, namely protons (carrying a positive charge) and electrons (carrying a negative charge). Neutrons, the third type of fundamental particle found in atoms, carry no charge (neutral).
The standard unit of charge is the Coulomb (C), named after the French physicist Charles-Augustin de Coulomb. The charge of a single proton is approximately +1.602 x 10⁻¹⁹ C, and the charge of a single electron is approximately -1.602 x 10⁻¹⁹ C. The fact that these charges are equal in magnitude but opposite in sign is crucial to understanding charge neutrality and conservation.
Charge Quantization
A significant aspect of electric charge is its quantization. This means that charge exists in discrete units, multiples of the elementary charge (e ≈ 1.602 x 10⁻¹⁹ C). You cannot have half an electron's charge or any fractional multiple of the elementary charge. This quantization arises from the fact that all observable charges are made up of integer numbers of elementary particles.
The Principle of Charge Conservation
The principle of conservation of charge asserts that in any physical process, the total electric charge of an isolated system remains constant. This means that the net charge before an interaction equals the net charge after the interaction. This holds true regardless of the type of interaction involved, be it chemical reactions, nuclear reactions, or interactions involving elementary particles.
In simpler terms: If you have a system with a certain total charge, no matter what processes occur within that system (as long as no charge enters or leaves the system), the total charge will always remain the same.
Examples Illustrating Charge Conservation
Let's consider a few examples to illustrate the principle:
-
Chemical Reactions: In a chemical reaction, electrons are transferred between atoms, changing the charge of individual atoms or ions. However, the total charge of the system remains constant. For instance, the reaction between sodium (Na) and chlorine (Cl) to form sodium chloride (NaCl) involves the transfer of an electron from sodium to chlorine. The total charge before the reaction (neutral Na and neutral Cl) is zero, and the total charge after the reaction (Na⁺ and Cl⁻) remains zero.
-
Nuclear Reactions: Similar to chemical reactions, nuclear reactions also obey the principle of charge conservation. In radioactive decay, for example, the total charge of the parent nucleus equals the sum of the charges of the daughter nucleus and the emitted particle(s).
-
Particle-Antiparticle Annihilation: When a particle and its antiparticle (e.g., an electron and a positron) collide, they annihilate each other, converting their mass into energy in the form of photons. However, the total charge remains conserved. Since the electron has a charge of -e and the positron has a charge of +e, the total charge before annihilation is zero, and the total charge after annihilation (zero charge in the photons) also remains zero.
Experimental Evidence for Charge Conservation
The principle of charge conservation is not merely a theoretical postulate; it's a well-established law of physics supported by numerous experimental observations over many decades. Precise measurements in various experiments have consistently confirmed that charge is conserved in all known processes.
For example, experiments involving particle physics, where high-energy collisions create numerous particles, have repeatedly shown that the total charge before and after the collisions remains the same. Similarly, experiments in nuclear physics, studying radioactive decay and nuclear reactions, have also demonstrated the conservation of charge.
No credible experiment has ever violated the principle of charge conservation. This consistent experimental verification lends strong credence to the fundamental nature of this law.
Implications and Applications of Charge Conservation
The conservation of charge has far-reaching implications in various fields of science and technology:
-
Electromagnetism: Charge conservation is a crucial element in Maxwell's equations, which describe the behavior of electric and magnetic fields. These equations rely on the fact that charge cannot be created or destroyed.
-
Electronics: The functioning of all electronic devices relies on the movement of electric charge. The conservation of charge ensures that the flow of current in a circuit is consistent and predictable, enabling the design and operation of electronic devices.
-
Particle Physics: Charge conservation is a fundamental symmetry in the Standard Model of particle physics, which describes the fundamental constituents of matter and their interactions. This principle helps physicists understand and predict the behavior of elementary particles and their interactions.
-
Chemistry: Charge conservation is vital in understanding chemical bonding and reactions. The transfer or sharing of electrons between atoms to form chemical bonds adheres strictly to this principle.
-
Cosmology: The conservation of charge plays a role in cosmological models, as it influences the distribution and behavior of charged particles in the universe.
Charge Conservation vs. Other Conservation Laws
Charge conservation is closely related to other fundamental conservation laws in physics:
-
Conservation of Energy: Energy can neither be created nor destroyed, only transformed from one form to another. While distinct, energy conservation and charge conservation often work in tandem, as processes involving charge transfer typically also involve energy changes.
-
Conservation of Momentum: The total momentum of a closed system remains constant in the absence of external forces. Similar to energy, momentum conservation is often intertwined with charge conservation in various physical processes.
-
Conservation of Baryon Number and Lepton Number: These are additional conservation laws that apply specifically to certain classes of elementary particles. While not directly related to charge, they illustrate the broader principle of conservation laws in physics.
Beyond the Basics: Further Considerations
While the basic principle of charge conservation is straightforward, deeper exploration reveals more nuanced aspects:
-
Local Charge Conservation: This refers to the conservation of charge at every point in space and time, implying that charge is neither created nor destroyed locally. This is a more stringent form of the conservation principle.
-
Gauge Invariance: Charge conservation is closely linked to the concept of gauge invariance, a fundamental symmetry in theoretical physics. Gauge invariance is a mathematical property that reflects the fact that physical phenomena should not depend on the arbitrary choice of reference point for potential.
-
Quantum Field Theory: Quantum field theory (QFT) provides a more sophisticated framework for understanding charge conservation, describing charges as quantum fields that obey specific conservation laws.
Conclusion
The conservation of charge is a fundamental and robust principle of physics, deeply rooted in experimental observations and theoretical understanding. Its importance extends across various scientific disciplines, from the behavior of subatomic particles to the operation of everyday electronic devices. Understanding this principle is essential for comprehending the behavior of matter and energy in the universe. Further research continues to explore the nuances of charge conservation and its implications within the broader context of fundamental physical laws, solidifying its place as a keystone in our understanding of the cosmos.
Latest Posts
Latest Posts
-
What Is Study Of Flowers Called
Mar 19, 2025
-
What Is The Complement Of A 45 Degree Angle
Mar 19, 2025
-
What Is The Secondary Structure Of Dna
Mar 19, 2025
-
What Is The Multiples Of 40
Mar 19, 2025
-
Least Common Multiple Of 60 And 24
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conservation Of Charge . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
