Rank The Following Chemical Bonds According To Their Strength
Juapaving
Mar 23, 2025 · 6 min read
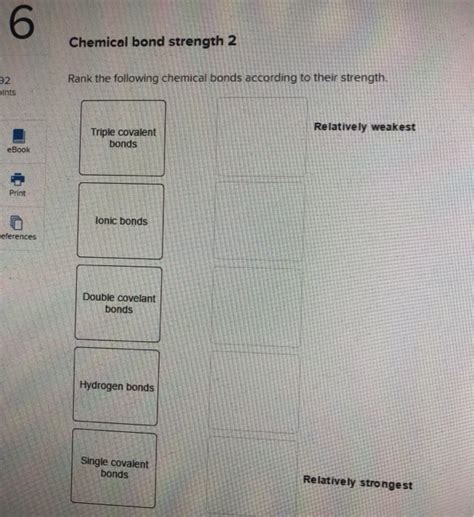
Table of Contents
Ranking Chemical Bonds by Strength: A Comprehensive Guide
Understanding the strength of chemical bonds is fundamental to chemistry. The strength of a bond dictates the properties of the molecules it forms, influencing everything from melting point and boiling point to reactivity and stability. This article comprehensively ranks various chemical bonds according to their strength, delving into the factors that contribute to bond strength and exploring real-world examples. We'll examine covalent bonds (including single, double, and triple bonds), ionic bonds, metallic bonds, and hydrogen bonds.
Factors Affecting Bond Strength
Before we delve into the ranking, let's understand the key factors determining a bond's strength:
1. Electrostatic Attraction: The primary force driving bond formation is the electrostatic attraction between oppositely charged particles. In ionic bonds, this is the attraction between positive and negative ions. In covalent bonds, it's the attraction between positively charged nuclei and shared negatively charged electrons. Stronger attractions lead to stronger bonds.
2. Bond Length: The distance between the nuclei of bonded atoms is crucial. Shorter bond lengths generally indicate stronger bonds because the electrostatic attraction is stronger at closer distances. This is inversely proportional; shorter distance means stronger attraction, stronger bond.
3. Bond Order: The number of electron pairs shared between two atoms is the bond order. Higher bond orders indicate stronger bonds. For example, a triple bond (bond order 3) is stronger than a double bond (bond order 2), which is stronger than a single bond (bond order 1). This is because more electrons are shared, resulting in greater electrostatic attraction.
4. Electronegativity: The electronegativity of an atom reflects its ability to attract electrons in a bond. A large difference in electronegativity between atoms often leads to polar bonds, where the electron density is unevenly distributed. While polarity itself doesn't directly determine strength, it can influence the overall stability and interactions within a molecule. Highly polar bonds can be more susceptible to certain reactions, indirectly affecting their apparent strength in specific contexts.
5. Atomic Size: Smaller atoms generally form stronger bonds because the shared electrons are closer to the positively charged nuclei. Larger atoms lead to longer bond lengths and weaker attractions.
Ranking Chemical Bonds: From Strongest to Weakest
Now, let's rank the major types of chemical bonds based on their typical strength. Keep in mind that these are general trends, and the exact strength can vary based on the specific atoms involved.
1. Covalent Bonds (Triple Bonds): These are generally the strongest type of chemical bond. The triple bond, with three shared electron pairs, results in a very strong electrostatic attraction. Examples include the nitrogen-nitrogen triple bond in N₂ (nitrogen gas) and the carbon-carbon triple bond in alkynes. The incredibly strong N≡N bond is responsible for the inertness of nitrogen gas under standard conditions.
2. Covalent Bonds (Double Bonds): Double bonds, with two shared electron pairs, are stronger than single bonds but weaker than triple bonds. They are shorter than single bonds, leading to increased attraction. Examples include the carbon-carbon double bonds in alkenes and the carbon-oxygen double bonds in carbonyl groups (like ketones and aldehydes). The presence of a double bond significantly impacts the reactivity and shape of molecules.
3. Covalent Bonds (Single Bonds): These are the most common type of covalent bond, involving one shared electron pair. They are weaker than double and triple bonds. Single bonds are flexible and readily rotate, impacting the shapes of molecules they form. Examples include the carbon-carbon single bonds in alkanes and the carbon-hydrogen bonds in many organic molecules. The strength of a single bond varies depending on the atoms involved; a C-C bond is stronger than a C-H bond, for instance.
4. Ionic Bonds: Ionic bonds result from the electrostatic attraction between oppositely charged ions (cations and anions). While they can be quite strong, they are generally weaker than the strongest covalent bonds (triple bonds). The strength of an ionic bond depends on the charge of the ions and the distance between them. Larger charges and smaller distances lead to stronger bonds. Examples include the bonds in sodium chloride (NaCl) and magnesium oxide (MgO). The high melting points of ionic compounds reflect the strength of these bonds.
5. Metallic Bonds: These bonds occur in metals, where valence electrons are delocalized and shared among a "sea" of electrons. The strength of metallic bonds varies greatly depending on the metal; transition metals generally exhibit stronger metallic bonds than alkali metals. The strength of these bonds is responsible for the characteristic properties of metals such as conductivity and malleability. The electron mobility allows metals to conduct electricity and heat efficiently.
6. Hydrogen Bonds: Hydrogen bonds are relatively weak compared to other types of chemical bonds, arising from the electrostatic attraction between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom. These bonds are crucial in many biological systems, influencing protein folding, DNA structure, and water properties. While individually weak, the cumulative effect of numerous hydrogen bonds can be significant. The relatively high boiling point of water is largely attributed to the strong hydrogen bonding network between water molecules.
Illustrative Examples and Applications
The strength of chemical bonds has profound implications across various fields:
-
Material Science: The strength of covalent bonds in diamond makes it one of the hardest known materials. The strong metallic bonds in steel contribute to its high tensile strength.
-
Biology: The strength and specificity of hydrogen bonds are vital for DNA replication and protein function. The covalent bonds within biomolecules dictate their stability and reactivity.
-
Chemistry: Understanding bond strengths is crucial for predicting reaction rates and mechanisms. Stronger bonds require more energy to break, making reactions involving them slower.
-
Pharmacology: The strength of bonds between drug molecules and their target receptors influences drug efficacy and duration of action.
-
Energy Production: Strong covalent bonds in fuels like hydrocarbons store significant chemical energy that is released upon combustion.
Conclusion: A Dynamic Spectrum
While we've presented a general ranking of chemical bonds by strength, it's crucial to remember that this is a simplification. The actual strength of a bond is highly dependent on the specific atoms involved, their environment, and other factors. The table below summarizes the ranking:
| Bond Type | Strength | Example | Notes |
|---|---|---|---|
| Covalent (Triple) | Very Strong | N₂ | Extremely strong bond due to three shared electron pairs. |
| Covalent (Double) | Strong | C=C (Alkenes) | Stronger than single bonds due to two shared electron pairs. |
| Covalent (Single) | Moderate | C-C (Alkanes) | Varies significantly based on the atoms involved. |
| Ionic | Moderate | NaCl | Strength depends on charge and distance between ions. |
| Metallic | Variable | Iron (Fe) | Strength varies greatly depending on the metal; generally moderate to strong |
| Hydrogen | Weak | Water (H₂O) | Individually weak but collectively significant in biological systems. |
This detailed overview provides a foundation for understanding the complexities of chemical bonding and its far-reaching implications across various scientific disciplines. Remember that the strength of a chemical bond isn't a static property but rather a dynamic interplay of multiple factors. Further investigation into specific bond types and molecular structures is recommended for a more nuanced understanding.
Latest Posts
Latest Posts
-
What Are The Multiples Of 63
Mar 24, 2025
-
Lcm Of 5 10 And 3
Mar 24, 2025
-
What Are The Least Common Multiples Of 8 And 12
Mar 24, 2025
-
What Is The Difference Between Psychology And Philosophy
Mar 24, 2025
-
Square Root Of 112 In Radical Form
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Rank The Following Chemical Bonds According To Their Strength . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
