One Similarity Between All Mixtures And Compounds Is That Both
Juapaving
Apr 01, 2025 · 6 min read
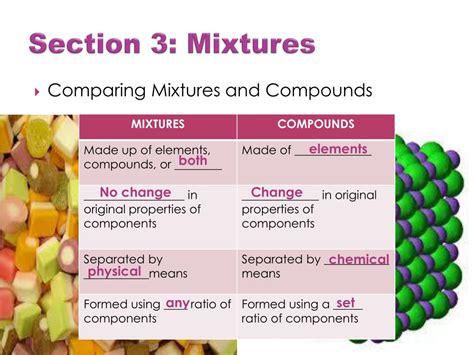
Table of Contents
The Universal Truth: All Mixtures and Compounds are Composed of Multiple Substances
One fundamental similarity unites all mixtures and compounds: they are both composed of multiple substances. While seemingly simple, this shared characteristic underpins a deeper understanding of matter and its diverse forms. This exploration delves into the intricacies of mixtures and compounds, highlighting their common ground and crucial distinctions. We will examine the fundamental building blocks, explore the diverse types of mixtures and compounds, and discuss the implications of this unifying characteristic in various scientific fields.
Understanding the Building Blocks: Atoms, Molecules, and Ions
Before diving into the specifics of mixtures and compounds, it's crucial to establish a foundational understanding of the building blocks of matter: atoms, molecules, and ions. Atoms are the fundamental units of elements, the simplest forms of matter that cannot be broken down by chemical means. Elements, such as oxygen (O), hydrogen (H), and carbon (C), are listed in the periodic table.
Molecules are formed when two or more atoms chemically bond, sharing or transferring electrons. Water (H₂O), for example, is a molecule composed of two hydrogen atoms and one oxygen atom bonded together. This bond is a strong chemical bond. The properties of a molecule are often significantly different from the properties of its constituent atoms.
Ions, on the other hand, are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge. For instance, a sodium ion (Na⁺) has lost one electron, giving it a positive charge, while a chloride ion (Cl⁻) has gained one electron, resulting in a negative charge. Ionic compounds are formed through the electrostatic attraction between positively and negatively charged ions.
Mixtures: A Heterogeneous Blend of Substances
Mixtures are physical combinations of two or more substances where the individual substances retain their chemical identities. Unlike compounds, mixtures are not formed through chemical reactions, meaning the components can be separated by physical means such as filtration, distillation, or evaporation. Crucially, the properties of a mixture are often a blend of the properties of its constituent components.
There are two primary types of mixtures:
1. Homogeneous Mixtures: Uniformity at the Macroscopic Level
Homogeneous mixtures, also known as solutions, exhibit a uniform composition throughout. This means that at the macroscopic level (visible to the naked eye), the mixture appears to be a single substance. Examples include saltwater (salt dissolved in water), air (a mixture of gases like oxygen, nitrogen, and carbon dioxide), and sugar dissolved in tea. The components of a homogeneous mixture are evenly distributed, meaning the properties are consistent throughout the sample.
2. Heterogeneous Mixtures: Visible Differences in Composition
Heterogeneous mixtures have a non-uniform composition. This means that different parts of the mixture have different properties. Examples include sand and water, oil and water, and a salad. You can easily visually distinguish the different components in a heterogeneous mixture. These components may settle or separate over time, due to differences in density or other physical properties.
Compounds: A Chemical Union of Elements
Compounds, unlike mixtures, are formed through chemical reactions. They are chemical combinations of two or more elements in fixed proportions. The elements in a compound lose their individual properties and combine to form a new substance with unique characteristics. This is a key distinction from mixtures. For example, water (H₂O) is a compound composed of hydrogen and oxygen, but it exhibits properties drastically different from either of its constituent elements. It is a liquid at room temperature, unlike hydrogen and oxygen which are gases.
The chemical bonds holding a compound together are strong and require chemical processes to break them apart. This means that the components of a compound cannot be separated by simple physical means. This is another significant difference from mixtures.
Types of Compounds: A Diverse Chemical Landscape
Compounds exhibit a vast array of properties and structures, which vary based on the elements involved and the nature of the bonds formed. Several important types include:
-
Ionic Compounds: Formed through the electrostatic attraction between oppositely charged ions. Table salt (NaCl) is a classic example, composed of positively charged sodium ions and negatively charged chloride ions.
-
Covalent Compounds: Formed by the sharing of electrons between atoms. Water (H₂O) is a quintessential example, with shared electrons forming covalent bonds between the hydrogen and oxygen atoms.
-
Metallic Compounds: Formed by the metallic bonding between metal atoms. These compounds exhibit unique properties such as high electrical and thermal conductivity.
-
Organometallic Compounds: Contain both metal and carbon atoms. They find wide application in various fields including catalysis.
The Unifying Characteristic: Composition of Multiple Substances
Returning to the central theme, the undeniable similarity between mixtures and compounds is their composition of multiple substances. Mixtures combine substances physically, while compounds combine substances chemically. However, both ultimately involve multiple entities. This shared characteristic is fundamental to our understanding of the macroscopic world. It is essential in various scientific and engineering fields.
Applications and Implications Across Disciplines
This fundamental similarity has profound implications across various fields:
1. Chemistry: Unveiling the Structure and Behavior of Matter
In chemistry, understanding the difference between mixtures and compounds is crucial for analyzing the composition of substances, predicting their reactivity, and designing new materials. The analysis techniques used differ greatly depending on whether a sample is a mixture or a compound. The separation techniques mentioned above are pivotal in isolating components from mixtures.
2. Material Science: Designing Novel Materials with Desired Properties
Material scientists utilize the principles of mixtures and compounds to design materials with specific properties. For example, alloys (mixtures of metals) are created by combining different metals to enhance strength, durability, or other desired properties. The controlled combination of elements in compounds allows for the creation of materials with tailored optical, electrical, and magnetic properties.
3. Environmental Science: Assessing Environmental Impacts
Understanding mixtures and compounds is critical for assessing the impact of pollutants on the environment. For instance, analyzing air and water samples for pollutants often requires differentiating between mixtures of various pollutants and the formation of new compounds due to chemical reactions between pollutants. This helps determine the risks associated with specific contaminants.
4. Biology: Understanding the Composition of Living Systems
Biological systems are incredibly complex mixtures and compounds. Living organisms are composed of a vast array of organic molecules (compounds containing carbon atoms) and inorganic molecules, all interacting in intricate ways. Understanding these mixtures and compounds is fundamental to understanding cellular function and the processes of life.
5. Geology: Studying the Composition of Rocks and Minerals
Rocks and minerals are complex mixtures and compounds of various elements and minerals. Analyzing their compositions is crucial in geological studies. The identification of various minerals within rocks provides clues about the geological history and formation processes.
Conclusion: A Foundation for Deeper Understanding
The shared characteristic of being composed of multiple substances underlies both mixtures and compounds. While their formation processes differ fundamentally—physical combination versus chemical reaction—both are essential for understanding the diversity and complexity of matter. This seemingly simple truth serves as a cornerstone for deeper investigations in chemistry, material science, environmental science, biology, and geology. Appreciating this unifying feature enhances our ability to analyze, manipulate, and utilize the myriad forms of matter found in our world. Further exploration into the specifics of mixtures and compounds will reveal the intricacies of the macroscopic world and its constituent components.
Latest Posts
Latest Posts
-
The Most Abundant Gas In The Earths Atmosphere Is
Apr 02, 2025
-
Is Rubbing Alcohol And Denatured Alcohol The Same
Apr 02, 2025
-
Is 17 A Prime Number Or A Composite Number
Apr 02, 2025
-
Is Burning A Candle A Chemical Or Physical Change
Apr 02, 2025
-
What Is The State Of Matter Of Fire
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about One Similarity Between All Mixtures And Compounds Is That Both . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
