Nitric Acid And Sodium Hydroxide Balanced Equation
Juapaving
Apr 01, 2025 · 6 min read
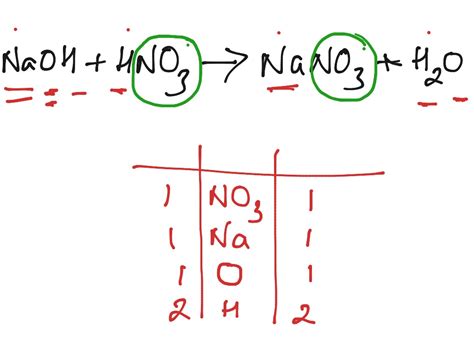
Table of Contents
Nitric Acid and Sodium Hydroxide: A Deep Dive into the Balanced Equation and its Implications
The reaction between nitric acid (HNO₃) and sodium hydroxide (NaOH) is a classic example of a neutralization reaction, a fundamental concept in chemistry. Understanding this reaction, its balanced equation, and its implications is crucial for various applications, from industrial processes to everyday life. This comprehensive article will explore the reaction in detail, covering its stoichiometry, the resulting products, safety precautions, and its relevance across different fields.
Understanding the Reactants
Before diving into the reaction itself, let's briefly review the properties of the two reactants: nitric acid and sodium hydroxide.
Nitric Acid (HNO₃)
Nitric acid is a strong, highly corrosive acid. It's a colorless liquid in its pure form, but often appears yellowish due to the presence of dissolved nitrogen oxides. Its strong acidic nature stems from its ability to readily donate a proton (H⁺) in aqueous solutions. Nitric acid is widely used in various industrial processes, including the production of fertilizers, explosives, and plastics. Its corrosive nature necessitates careful handling and appropriate safety measures.
Key properties of Nitric Acid:
- Strong acid: Completely dissociates in water.
- Oxidizing agent: Can readily accept electrons, leading to redox reactions.
- Corrosive: Damages many materials, including metals and skin.
- Hazardous: Requires careful handling and storage.
Sodium Hydroxide (NaOH)
Sodium hydroxide, also known as caustic soda or lye, is a strong, highly corrosive base. It's a white crystalline solid that readily dissolves in water, releasing a significant amount of heat in the process (an exothermic reaction). Its strong basicity arises from its ability to readily accept protons (H⁺). Sodium hydroxide finds extensive use in various applications, such as soap making, paper production, and drain cleaning. Similar to nitric acid, its corrosive nature mandates careful handling and safety protocols.
Key properties of Sodium Hydroxide:
- Strong base: Completely dissociates in water.
- Highly corrosive: Damages many materials, including skin and eyes.
- Delliquescent: Absorbs moisture from the air.
- Hazardous: Requires careful handling and storage.
The Neutralization Reaction: HNO₃ + NaOH
When nitric acid and sodium hydroxide react, they undergo a neutralization reaction, producing water and a salt. This is a classic acid-base reaction where the hydrogen ions (H⁺) from the acid react with the hydroxide ions (OH⁻) from the base to form water (H₂O). The remaining ions combine to form the salt, in this case, sodium nitrate (NaNO₃).
The Balanced Chemical Equation
The balanced chemical equation for the reaction between nitric acid and sodium hydroxide is:
HNO₃(aq) + NaOH(aq) → NaNO₃(aq) + H₂O(l)
This equation signifies that one mole of nitric acid reacts with one mole of sodium hydroxide to produce one mole of sodium nitrate and one mole of water. The "(aq)" indicates that the substance is dissolved in water (aqueous solution), while "(l)" denotes a liquid state. The equation is balanced because the number of atoms of each element is the same on both the reactant and product sides.
Stoichiometry and Calculations
The balanced equation allows us to perform stoichiometric calculations. For example, if we know the amount of nitric acid used, we can calculate the amount of sodium hydroxide required for complete neutralization, or vice versa. This is crucial in titrations, a common analytical technique used to determine the concentration of an unknown solution.
Example:
Let's say we have 50 mL of 0.1 M nitric acid. How many mL of 0.1 M sodium hydroxide are needed for complete neutralization?
Using the balanced equation, we know that the mole ratio of HNO₃ to NaOH is 1:1. First, we calculate the moles of HNO₃:
Moles of HNO₃ = (Volume in L) × (Molarity) = (0.050 L) × (0.1 mol/L) = 0.005 moles
Since the mole ratio is 1:1, we need 0.005 moles of NaOH. Now we can calculate the volume of 0.1 M NaOH:
Volume of NaOH = (Moles) / (Molarity) = (0.005 moles) / (0.1 mol/L) = 0.050 L = 50 mL
Therefore, 50 mL of 0.1 M sodium hydroxide is required to completely neutralize 50 mL of 0.1 M nitric acid.
Properties of the Products
The products of the reaction, sodium nitrate (NaNO₃) and water (H₂O), have significantly different properties than the reactants.
Sodium Nitrate (NaNO₃)
Sodium nitrate is a soluble salt, meaning it readily dissolves in water. It's a white crystalline solid and is commonly used as a fertilizer, food preservative (E251), and in the manufacture of other chemicals. Unlike the reactants, it's relatively non-corrosive and non-hazardous.
Water (H₂O)
Water is the universal solvent and is essential for life. Its formation in this neutralization reaction is an indication of the completion of the acid-base reaction. The heat released during this reaction is absorbed by the water, causing a slight increase in temperature.
Safety Precautions
Both nitric acid and sodium hydroxide are highly corrosive and require careful handling. Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat. The reaction itself generates heat, so it should be carried out in a well-ventilated area or under a fume hood to prevent inhalation of any fumes. Spills should be cleaned up immediately using appropriate neutralizing agents and following established safety protocols.
Applications and Relevance
The neutralization reaction between nitric acid and sodium hydroxide, while seemingly simple, has several crucial applications across various fields:
- Industrial Chemistry: Neutralization reactions are used extensively in industrial processes to control pH levels, treat wastewater, and in the production of various chemicals.
- Analytical Chemistry: Titrations, based on neutralization reactions, are fundamental in determining the concentration of unknown acids or bases.
- Environmental Science: Understanding neutralization reactions is essential for managing environmental pollution, particularly acid rain.
- Food Science: Controlled neutralization is used in food processing to adjust pH levels and in the production of certain food additives.
Beyond the Basics: Exploring Further Concepts
The simple neutralization reaction provides a foundation for understanding more complex chemical processes. Factors such as temperature, concentration, and the presence of other substances can influence the reaction rate and equilibrium. Furthermore, exploring the thermodynamics of the reaction, including enthalpy changes (heat released or absorbed), provides a deeper understanding of the energy involved.
Conclusion
The reaction between nitric acid and sodium hydroxide is a fundamental example of a neutralization reaction, offering valuable insights into acid-base chemistry. Understanding its balanced equation, stoichiometry, and the properties of the reactants and products is crucial for various applications. Always remember the importance of safety precautions when handling corrosive chemicals like nitric acid and sodium hydroxide. This comprehensive exploration provides a solid foundation for further study and applications in diverse scientific and industrial fields. The balanced equation's simplicity belies its far-reaching importance in numerous chemical processes and analytical techniques. Remember to always prioritize safety when working with these chemicals.
Latest Posts
Latest Posts
-
How To Find Recoil In Physics
Apr 02, 2025
-
87 Inches Is How Many Feet
Apr 02, 2025
-
Electrons Are Lost Or Gained During
Apr 02, 2025
-
What Are The 3 Components Of A Nucleotide
Apr 02, 2025
-
What Are Non Living Parts Of The Environment Called
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Nitric Acid And Sodium Hydroxide Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
