Methods Of Purification In Organic Chemistry
Juapaving
Mar 17, 2025 · 7 min read
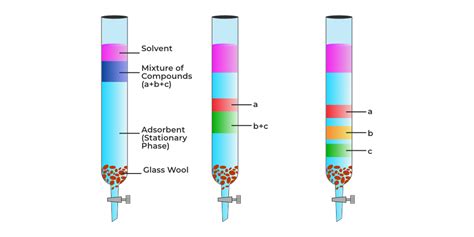
Table of Contents
Methods of Purification in Organic Chemistry
Organic chemistry, the study of carbon-containing compounds, relies heavily on the ability to isolate and purify the desired product from a complex reaction mixture. The purity of a compound is crucial for accurate characterization and reliable experimental results. Numerous methods exist for purifying organic compounds, each with its strengths and limitations depending on the physical and chemical properties of the target molecule and the impurities present. This article delves into the various methods of purification in organic chemistry, exploring their principles, applications, and limitations.
I. Physical Methods of Purification
These methods exploit the differences in physical properties like boiling point, melting point, solubility, and volatility to separate components of a mixture.
A. Recrystallization
Recrystallization is a powerful technique for purifying solid organic compounds. It leverages the difference in solubility of the compound at different temperatures. The impure solid is dissolved in a hot solvent, forming a saturated solution. As the solution cools, the solubility of the compound decreases, leading to the crystallization of the purified compound while impurities remain dissolved in the solution or are excluded from the crystal lattice.
Key Considerations:
- Solvent Selection: The ideal solvent should readily dissolve the compound at high temperatures but poorly at low temperatures. It should also dissolve the impurities readily or not at all. Common solvents include ethanol, methanol, water, and hexane.
- Seed Crystal: Sometimes, adding a small seed crystal of the pure compound can initiate crystallization.
- Filtration: After crystallization, the purified crystals are separated from the mother liquor by filtration (gravity or vacuum).
- Drying: The crystals are then dried to remove residual solvent.
Advantages: Relatively simple, highly effective for removing soluble impurities.
Limitations: Not suitable for compounds that decompose on heating or that are difficult to crystallize. Loss of product is inevitable.
B. Distillation
Distillation separates liquids based on their boiling points. The mixture is heated, and the component with the lowest boiling point vaporizes first, then condenses in a separate collection vessel. Several variations of distillation exist to enhance separation efficiency.
- Simple Distillation: Suitable for separating liquids with significantly different boiling points (> 70°C difference).
- Fractional Distillation: Utilizes a fractionating column to enhance separation of liquids with closer boiling points. The column provides multiple condensation-vaporization cycles, leading to better separation.
- Steam Distillation: Used for isolating volatile compounds that are immiscible with water. Steam is passed through the mixture, carrying the volatile compound into the distillate.
- Vacuum Distillation: Employs reduced pressure to lower the boiling points of high-boiling liquids, preventing decomposition.
Advantages: Highly effective for separating volatile liquids.
Limitations: Less effective for liquids with similar boiling points; can be time-consuming.
C. Extraction
Extraction separates compounds based on their relative solubility in two immiscible solvents. The mixture is shaken with a solvent in which the desired compound is more soluble. The two layers are then separated, and the desired compound is recovered from the extracting solvent.
- Liquid-Liquid Extraction: The most common type, using two immiscible solvents like water and an organic solvent (e.g., diethyl ether, dichloromethane).
- Acid-Base Extraction: Used to separate acidic, basic, and neutral compounds. Acidic compounds are extracted with a base, basic compounds with an acid, and neutral compounds remain in the organic layer.
Advantages: Effective for separating compounds with different polarities and acid-base properties.
Limitations: Requires careful selection of solvents and can be time-consuming. Some loss of product is inevitable due to incomplete extraction.
D. Chromatography
Chromatography is a powerful separation technique based on the differential distribution of compounds between a stationary phase and a mobile phase. Various types of chromatography exist, each utilizing different principles.
-
Thin-Layer Chromatography (TLC): A simple and rapid technique using a thin layer of adsorbent (e.g., silica gel) coated on a plate. The mixture is spotted onto the plate, and the mobile phase moves up the plate by capillary action, separating the components based on their affinity for the stationary and mobile phases. Used for qualitative analysis and monitoring reaction progress.
-
Column Chromatography: A larger-scale version of TLC, using a column packed with an adsorbent. The mixture is added to the top of the column, and the mobile phase is passed through, separating the components based on their affinity for the stationary and mobile phases. Used for preparative purification.
-
High-Performance Liquid Chromatography (HPLC): A sophisticated technique using high pressure to force the mobile phase through a tightly packed column. Offers superior resolution and is widely used for quantitative analysis and purification of complex mixtures.
-
Gas Chromatography (GC): Used to separate volatile compounds. The mixture is vaporized and carried through a column by an inert gas (e.g., helium). Components are separated based on their boiling points and interactions with the stationary phase. Often coupled with a mass spectrometer (GC-MS) for identification.
Advantages: Highly versatile, effective for separating complex mixtures.
Limitations: Can be expensive and time-consuming, especially for HPLC and GC.
II. Chemical Methods of Purification
These methods employ chemical reactions to remove impurities or convert the desired compound into a more easily purified derivative.
A. Derivatization
Derivatization involves converting the compound of interest into a derivative with different physical properties, making it easier to purify. After purification, the derivative is converted back to the original compound. For example, carboxylic acids can be converted to their methyl esters, which are often easier to purify by distillation.
Advantages: Can improve the ease of purification for difficult-to-purify compounds.
Limitations: Requires additional steps and can introduce new impurities.
B. Washing
Washing involves removing impurities by treating the crude product with a suitable solvent. For instance, washing an organic solution with water can remove water-soluble impurities. Alternatively, an acidic or basic wash can remove impurities with opposing properties.
Advantages: Simple and efficient for removing certain types of impurities.
Limitations: Only effective for removing readily soluble impurities.
C. Sublimation
Sublimation is a purification technique applicable to solids that can pass directly from the solid phase to the gaseous phase without melting. The impure solid is heated gently, and the purified compound sublimes, then deposits on a cool surface, leaving impurities behind.
Advantages: Effective for purifying solids that sublime easily.
Limitations: Not applicable to all compounds; can be slow.
III. Choosing the Right Purification Method
Selecting the optimal purification method depends on several factors:
- Nature of the compound: Solid or liquid, boiling point, melting point, solubility, stability, polarity.
- Type and amount of impurities: Soluble or insoluble, acidic, basic, or neutral.
- Scale of purification: Small-scale laboratory work or large-scale industrial production.
- Availability of equipment and resources: Access to sophisticated instruments like HPLC or GC.
Often, a combination of techniques is employed to achieve the desired level of purity. For example, recrystallization may be followed by sublimation or distillation. Careful consideration of the compound's properties and the available resources are crucial for selecting the most effective and efficient purification strategy.
IV. Characterization of Purity
After purification, it's crucial to verify the purity of the obtained compound. Several techniques are employed for this purpose:
- Melting Point Determination: A sharp melting point over a narrow range indicates high purity.
- Boiling Point Determination: Similar to melting point, a sharp boiling point suggests high purity.
- Spectroscopic Analysis: Techniques like NMR, IR, and UV-Vis spectroscopy provide detailed information about the structure and purity of the compound.
- Chromatographic Analysis: TLC, HPLC, and GC can reveal the presence of impurities and assess the purity of the sample.
The methods discussed above represent the core purification techniques used in organic chemistry. The choice of the best method is determined by a careful analysis of the properties of the target compound and the impurities present. Understanding the principles and limitations of each technique is critical for successfully purifying organic compounds and ensuring the accuracy and reliability of experimental results. Furthermore, proper safety precautions should always be followed when handling chemicals and performing purification procedures.
Latest Posts
Latest Posts
-
66 As A Product Of Prime Factors
Mar 18, 2025
-
Vertical And Horizontal Lines On A Graph
Mar 18, 2025
-
Lcm Of 8 12 And 15
Mar 18, 2025
-
Is 55 A Prime Or Composite Number
Mar 18, 2025
-
What Are The Characteristics Of Igneous Rocks
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Methods Of Purification In Organic Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
