Liquids At Room Temperature Periodic Table
Juapaving
Mar 29, 2025 · 6 min read
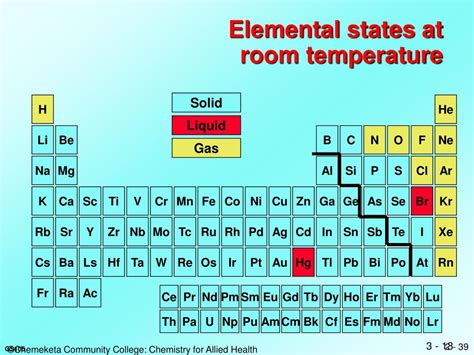
Table of Contents
Liquids at Room Temperature: A Periodic Table Perspective
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While many elements exist as solids at room temperature, a fascinating subset exists as liquids. Understanding which elements are liquid at room temperature and why requires exploring their chemical and physical properties, offering a unique lens through which to view the periodic table itself. This article delves into the intriguing world of elements that defy the norm and remain liquid under standard conditions, exploring their unique characteristics and applications.
The Rarity of Liquid Elements at Room Temperature
Most elements on the periodic table are solids at room temperature (around 25°C or 77°F). This is largely due to strong interatomic forces holding their atoms tightly together in a crystalline structure. The transition to a liquid state requires sufficient energy to overcome these forces, allowing atoms to move more freely. Therefore, finding elements liquid at room temperature is relatively rare. Only six elements achieve this remarkable feat under standard atmospheric pressure:
- Mercury (Hg): A heavy, silvery-white liquid metal, renowned for its toxicity and historical use in thermometers and barometers.
- Bromine (Br): A reddish-brown, volatile liquid nonmetal, known for its pungent odor and corrosive nature.
- Francium (Fr): A highly radioactive, extremely rare alkali metal. Its extreme radioactivity and short half-life make studying its liquid state incredibly challenging.
- Cesium (Cs): A highly reactive alkali metal that melts slightly above room temperature.
- Gallium (Ga): A silvery-white metal with an unusually low melting point, often exhibiting a peculiar “melting in the hand” characteristic.
- Rubidium (Rb): Another highly reactive alkali metal with a melting point slightly above room temperature.
This limited number highlights the specific atomic and molecular characteristics required for liquid state existence at room temperature. Let's investigate these individual elements more deeply.
Detailed Examination of Liquid Elements at Room Temperature
1. Mercury (Hg): The Liquid Metal
Mercury, with its atomic number 80, stands as the only metallic element that remains liquid at room temperature. Its unique properties stem from its electronic configuration and strong relativistic effects. The inner electrons move at speeds comparable to the speed of light, leading to a contraction of the 6s orbital. This contraction weakens the metallic bonding, resulting in a relatively low melting point and high volatility compared to other transition metals.
Applications: While its toxicity is now widely recognized, mercury historically found extensive applications, including:
- Thermometers and Barometers: Utilizing its thermal expansion properties.
- Electrical Switches and Relays: Leveraging its high electrical conductivity.
- Fluorescent Lamps: As a component in the generation of ultraviolet (UV) light.
However, due to its severe environmental and health risks, mercury's usage is significantly restricted and replaced by safer alternatives whenever possible.
2. Bromine (Br): The Reactive Nonmetal
Bromine, atomic number 35, is the only nonmetal liquid at room temperature. This reddish-brown liquid is highly reactive, readily forming compounds with metals and other elements. Its relatively weak intermolecular forces compared to other halogens allow it to exist as a liquid at room temperature. However, its volatility makes handling it carefully necessary to prevent vapor inhalation, causing respiratory irritation.
Applications:
- Production of flame retardants: Brominated flame retardants were used in various products but are facing increasing restrictions due to environmental concerns.
- Disinfectants and pesticides: Bromine's reactive nature makes it suitable for these applications, although safer alternatives are increasingly preferred.
- Organic synthesis: It serves as a useful reagent in the synthesis of various organic compounds.
3. Francium (Fr), Cesium (Cs), Rubidium (Rb): The Alkali Metal Trio
Francium, cesium, and rubidium, belonging to Group 1 (alkali metals), exhibit low melting points due to their weak metallic bonding. Their outermost electron is loosely held, leading to low ionization energies and high reactivity. This reactivity makes handling them extremely challenging and necessitates specialized conditions. While cesium and rubidium can be considered liquids near room temperature, Francium’s extreme radioactivity and short half-life prevent any meaningful consideration of its properties under standard conditions. The extreme reactivity of all three limits their practical applications to specialized scientific research.
Applications (primarily Cs and Rb):
- Atomic clocks: Cesium clocks, for example, are highly accurate due to cesium's distinct spectral lines.
- Photoelectric cells: Cesium's low ionization energy makes it useful in photoelectric devices.
- Scientific research: These elements find use in various research areas, including spectroscopy and nuclear physics.
4. Gallium (Ga): The Melting Metal
Gallium, atomic number 31, stands out for its unusually low melting point (29.76 °C), just above room temperature. This low melting point is attributed to its unique crystal structure and weak metallic bonding. Its propensity to melt in the hand is a striking characteristic often used in demonstrations.
Applications:
- Semiconductors: Gallium arsenide (GaAs) is a crucial semiconductor material used in high-speed electronic devices and solar cells.
- LEDs: Gallium nitride (GaN) is used in the production of high-efficiency light-emitting diodes.
- Medical applications: Gallium-based compounds find uses in medical imaging and cancer treatment.
Factors Affecting the Liquid State at Room Temperature
Several factors determine whether an element will be a liquid at room temperature:
- Atomic size and mass: Larger atoms generally exhibit weaker interatomic forces, favoring liquid states.
- Metallic bonding strength: Weak metallic bonding, like in alkali metals, leads to lower melting points.
- Intermolecular forces: For nonmetals, the strength of intermolecular forces (like van der Waals forces) influences the melting point.
- Relativistic effects: In heavy elements like mercury, relativistic effects play a significant role in determining the electron configuration and bonding strength.
The Periodic Table and Liquid Elements: Trends and Patterns
Examining the periodic table, we can observe some trends regarding the liquid elements:
- Group 1 (Alkali Metals): The heavier alkali metals (cesium and rubidium) have low melting points, nearing or exceeding room temperature.
- Group 17 (Halogens): Bromine is the only halogen that is liquid at room temperature.
- Transition Metals: Mercury is the only transition metal liquid at room temperature, highlighting its unique electronic configuration and relativistic effects.
- Post-transition Metals: Gallium, a post-transition metal, displays an exceptionally low melting point.
Future Discoveries and Research
While the list of elements liquid at room temperature currently stands at six, further research might unveil unexpected discoveries, especially with the synthesis and study of new and exotic materials. The exploration of extreme conditions, such as high pressure, could also alter the phase behavior of elements, possibly leading to additional instances of liquid elements under these non-standard conditions.
Conclusion: Liquids at Room Temperature—Exceptions That Enrich Our Understanding
The relatively small number of elements liquid at room temperature makes them notable exceptions within the broader context of the periodic table. Their unique physical and chemical properties offer valuable insights into atomic structure, bonding, and intermolecular forces. Understanding why these elements defy the typical solid state at ambient conditions provides a deeper appreciation of the intricate relationships between an element's atomic characteristics and its macroscopic behavior. Furthermore, the diverse applications of these elements, ranging from everyday thermometers to cutting-edge semiconductor technology, demonstrate their significance in various scientific and technological fields, even while the risks associated with their toxicity and reactivity mandate careful handling and safe alternatives when possible. The continued study of these remarkable elements will undoubtedly lead to further discoveries and advancements in various scientific and technological areas.
Latest Posts
Latest Posts
-
Is A Prime Number Even Or Odd
Apr 01, 2025
-
Least Common Multiple 12 And 16
Apr 01, 2025
-
Ionic Compounds Are Composed Of What Particles
Apr 01, 2025
-
What Is 3 33333 As A Fraction
Apr 01, 2025
-
What Does A Prokaryotic Cell Not Have
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Liquids At Room Temperature Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
