Ionic Compounds Are Composed Of What Particles
Juapaving
Apr 01, 2025 · 6 min read
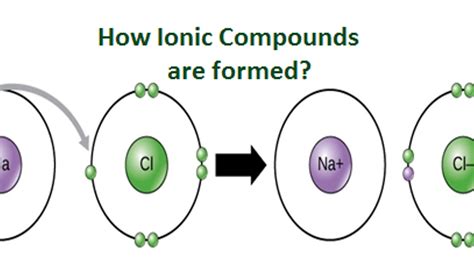
Table of Contents
Ionic Compounds: A Deep Dive into Their Composition
Ionic compounds are fundamental building blocks of chemistry, ubiquitous in our world and essential to countless processes. Understanding their composition is key to grasping their properties and behavior. This article will explore the fundamental particles that make up ionic compounds, delving into the intricacies of their formation, structure, and properties. We'll examine the role of electrons, ions, and the electrostatic forces that govern these fascinating substances.
The Building Blocks: Ions – Positively and Negatively Charged Particles
The cornerstone of any ionic compound is the ion. Unlike neutral atoms, ions carry a net electrical charge. This charge arises from an imbalance in the number of protons (positively charged) and electrons (negatively charged). There are two main types of ions:
Cations: Positively Charged Ions
Cations are formed when an atom loses one or more electrons. This loss typically occurs when an atom has a relatively low ionization energy, meaning it doesn't require a lot of energy to remove an electron. Metals, particularly those in Groups 1 and 2 of the periodic table (alkali and alkaline earth metals), readily form cations because they have relatively few electrons in their outermost shell (valence electrons). Losing these electrons achieves a stable electron configuration, often resembling a noble gas.
- Example: Sodium (Na) readily loses one electron to form a sodium cation (Na⁺). This is because losing that single electron leaves it with a full outer shell, mimicking the stable configuration of neon (Ne).
Anions: Negatively Charged Ions
Anions, conversely, are formed when an atom gains one or more electrons. Nonmetals, especially those in Groups 16 and 17 (chalcogens and halogens), have a strong tendency to gain electrons to complete their outer shell. This is because gaining electrons is energetically favorable, resulting in a more stable electronic configuration.
- Example: Chlorine (Cl) readily gains one electron to form a chloride anion (Cl⁻). This addition completes its outer shell, mirroring the stable configuration of argon (Ar).
The Formation of Ionic Compounds: Electrostatic Attraction
Ionic compounds are formed through a process called ionic bonding. This involves the strong electrostatic attraction between oppositely charged ions. The process typically unfolds as follows:
-
Electron Transfer: A metal atom (which readily loses electrons) transfers one or more electrons to a nonmetal atom (which readily gains electrons).
-
Ion Formation: The metal atom becomes a positively charged cation, while the nonmetal atom becomes a negatively charged anion.
-
Electrostatic Attraction: The opposite charges of the cation and anion attract each other strongly, forming an ionic bond. This attractive force is what holds the ions together in the ionic compound.
The strength of this electrostatic attraction depends on several factors:
-
Charge Magnitude: Higher charges (e.g., 2+ and 2−) lead to stronger attractions than lower charges (e.g., 1+ and 1−).
-
Ionic Radius: Smaller ions lead to stronger attractions because the charges are closer together. Larger ions result in weaker attractions due to greater distance between the charges.
The Structure of Ionic Compounds: Crystal Lattices
Ionic compounds don't exist as individual molecules; instead, they form crystal lattices. These are three-dimensional, repeating arrangements of ions, with cations and anions packed together in a way that maximizes electrostatic attraction and minimizes repulsion. The specific arrangement depends on the size and charge of the ions involved.
Different ionic compounds exhibit various crystal lattice structures, including:
-
Simple Cubic: A relatively simple arrangement, but not very efficient in packing ions.
-
Body-Centered Cubic: A more efficient arrangement than simple cubic, with ions at the corners and center of the cube.
-
Face-Centered Cubic: The most efficient packing arrangement, with ions at the corners and the center of each face of the cube.
The repeating nature of these lattices gives rise to the characteristic properties of ionic compounds, such as their crystalline structure and high melting points.
Properties of Ionic Compounds: A Reflection of Their Composition
The unique composition of ionic compounds—the strong electrostatic forces between ions arranged in a crystal lattice—results in several characteristic properties:
High Melting and Boiling Points
The strong electrostatic forces between ions require significant energy to overcome. Consequently, ionic compounds generally have high melting and boiling points compared to covalent compounds.
Brittleness
Ionic crystals are brittle because applying force can shift the layers of ions, bringing similarly charged ions into close proximity. This leads to strong repulsive forces, causing the crystal to fracture.
Conductivity
Ionic compounds generally conduct electricity when molten (liquid) or dissolved in water. In these states, the ions are free to move and carry an electric current. However, in their solid state, the ions are fixed in the lattice and cannot move freely to conduct electricity.
Solubility
The solubility of ionic compounds varies depending on the specific ions and the solvent. Polar solvents, such as water, tend to dissolve many ionic compounds because the polar water molecules can interact with the charged ions, weakening the electrostatic forces holding the crystal together.
Examples of Ionic Compounds and Their Constituent Ions
Let's examine some common examples to illustrate the principles discussed:
-
Sodium Chloride (NaCl): Composed of sodium cations (Na⁺) and chloride anions (Cl⁻).
-
Magnesium Oxide (MgO): Composed of magnesium cations (Mg²⁺) and oxide anions (O²⁻). Note the higher charges leading to stronger electrostatic attraction and a higher melting point compared to NaCl.
-
Potassium Iodide (KI): Composed of potassium cations (K⁺) and iodide anions (I⁻).
-
Calcium Chloride (CaCl₂): Composed of calcium cations (Ca²⁺) and chloride anions (Cl⁻). Note that the ratio of cations to anions is 1:2 to maintain electrical neutrality.
Beyond Simple Ionic Compounds: Polyatomic Ions
While the examples above feature monatomic ions (single atoms with a charge), many ionic compounds contain polyatomic ions. These are groups of atoms covalently bonded together that carry a net electrical charge.
Examples of common polyatomic ions include:
-
Nitrate (NO₃⁻): A group of one nitrogen atom and three oxygen atoms with a -1 charge.
-
Sulfate (SO₄²⁻): A group of one sulfur atom and four oxygen atoms with a -2 charge.
-
Ammonium (NH₄⁺): A group of one nitrogen atom and four hydrogen atoms with a +1 charge.
These polyatomic ions behave similarly to monatomic ions in the formation and properties of ionic compounds. For example, ammonium nitrate (NH₄NO₃) is an ionic compound composed of ammonium cations (NH₄⁺) and nitrate anions (NO₃⁻).
Applications of Ionic Compounds: A Wide Range of Uses
Ionic compounds play crucial roles in various applications:
-
Medicine: Many ionic compounds are used as medications or in medical treatments.
-
Agriculture: Fertilizers often contain ionic compounds to provide essential nutrients to plants.
-
Industry: Ionic compounds are employed in numerous industrial processes, from manufacturing to construction.
-
Everyday Life: Table salt (NaCl) is a ubiquitous example of an ionic compound found in most households.
Conclusion: Understanding the Foundation of Ionic Compounds
Ionic compounds are fascinating materials with properties directly stemming from their unique composition: the electrostatic attraction between positively charged cations and negatively charged anions arranged in a crystal lattice. This arrangement dictates their high melting points, brittleness, and conductivity in specific states. Understanding the fundamental particles—cations and anions—and the forces governing their interactions is vital to appreciating the widespread applications and crucial roles of these compounds in our world. The study of ionic compounds provides a cornerstone for understanding a wide range of chemical phenomena and their practical implications. From the simple salt on our table to complex industrial processes, the impact of ionic compounds is undeniable.
Latest Posts
Latest Posts
-
Magnetic Field From A Current Loop
Apr 02, 2025
-
Collection Of Nerve Cell Bodies Outside The Cns
Apr 02, 2025
-
What Do Nitrification And Denitrification Have In Common
Apr 02, 2025
-
What Is The Lowest Common Multiple Of 3 And 7
Apr 02, 2025
-
How To Find A Supplementary Angle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Ionic Compounds Are Composed Of What Particles . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
