Is Temperature An Extensive Or Intensive Property
Juapaving
Mar 16, 2025 · 5 min read
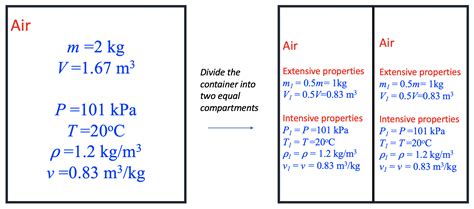
Table of Contents
Is Temperature an Extensive or Intensive Property? A Deep Dive
The question of whether temperature is an extensive or intensive property is a fundamental one in thermodynamics, often causing confusion among students and even seasoned scientists. Understanding this distinction is crucial for mastering various thermodynamic concepts and calculations. This article will delve deep into the nature of temperature, exploring its characteristics, contrasting it with extensive properties, and ultimately clarifying its classification. We'll also touch upon related concepts to provide a comprehensive understanding.
Understanding Extensive and Intensive Properties
Before we address the core question, let's establish a clear understanding of extensive and intensive properties. These terms describe how a property changes when the amount of substance is altered.
Extensive Properties: These properties depend on the size or amount of matter present. Doubling the amount of substance doubles the value of the extensive property. Examples include:
- Mass: The total mass of a system increases proportionally with the amount of matter.
- Volume: The volume occupied by a substance is directly related to its quantity.
- Energy: The total energy of a system is the sum of the energies of its components.
- Enthalpy: Similar to energy, enthalpy scales with the amount of substance.
- Entropy: A measure of disorder, entropy is also an extensive property.
Intensive Properties: These properties are independent of the amount of substance. They remain constant even if the amount of matter changes. Examples include:
- Temperature: This is the central focus of our discussion.
- Pressure: The pressure exerted by a gas doesn't change if you increase the amount of gas while keeping the volume constant.
- Density: The mass per unit volume remains the same regardless of the total mass.
- Concentration: The amount of solute per unit volume of solution is independent of the total volume.
- Boiling Point: The temperature at which a liquid boils is a characteristic property of the substance, not its amount.
Temperature: A Closer Look
Temperature is a measure of the average kinetic energy of the particles within a system. This kinetic energy is associated with the random motion of atoms and molecules. Higher temperatures imply higher average kinetic energy, meaning particles are moving faster and colliding more frequently.
It's crucial to distinguish between temperature and heat. Heat is the transfer of thermal energy between systems due to a temperature difference. Temperature, on the other hand, is a state function, representing the internal state of a system. This is a key factor in determining its classification.
Why Temperature is an Intensive Property
Temperature's independence from the system's size is what firmly places it in the category of intensive properties. Consider the following scenarios:
-
Two identical blocks of metal at the same temperature: If you combine these blocks, the temperature of the combined system remains the same. It doesn't increase simply because you have more metal. The average kinetic energy of the particles is unchanged.
-
Mixing two liquids of different temperatures: When you mix two liquids with different temperatures, the final temperature will be an intermediate value, a result of the thermal equilibrium process. The final temperature is not a simple sum of the individual temperatures. The final temperature is determined by the heat exchange and the heat capacity of each liquid, neither of which depends solely on the volume of liquid involved.
Contrasting Temperature with Extensive Properties
Let's directly compare temperature to a classic extensive property: volume.
If you have two identical containers filled with the same gas at the same temperature and pressure, the total volume of the gas is double that of a single container. The volume is directly proportional to the quantity of gas. However, if you combine those two containers, the temperature remains the same, proving its intensive nature. The individual containers and the combined system all have the same temperature.
This difference highlights a fundamental distinction: extensive properties are additive, while intensive properties are not. You can add volumes, but you cannot simply add temperatures.
Practical Applications and Examples
The intensive nature of temperature has significant implications in various fields:
-
Thermometry: Accurate temperature measurement relies on the fact that temperature is independent of the amount of the substance being measured. The thermometer's reading doesn't change significantly when the amount of substance in contact with the thermometer is increased.
-
Phase Transitions: The boiling and melting points of a substance are intensive properties. A small sample of water boils at the same temperature as a large body of water.
-
Chemical Reactions: Temperature is a critical factor influencing reaction rates and equilibrium constants. The temperature of the reaction mixture remains constant regardless of the scale of the reaction (within reasonable limits).
-
Heat Transfer: Understanding that temperature is intensive allows for accurate predictions of heat transfer rates between systems with different temperatures. The larger system will experience a smaller temperature change, while the smaller system experiences a larger change; however, the final temperature is still determined by heat capacity and specific heat of the individual substances rather than simply the magnitude of volume/mass.
Advanced Concepts and Clarifications
Some might argue that in specific situations, temperature might appear to be influenced by the amount of substance. For example, adding a small amount of cold water to a large body of hot water will result in a small decrease in the overall temperature. However, this is due to the transfer of heat energy, not a change in the inherent temperature of either volume of water. The temperature of the water remains constant before heat transfer occurs.
Furthermore, the concept of thermodynamic equilibrium is crucial. When different systems are brought into thermal contact, heat flows until thermal equilibrium is reached—a state where temperature is uniform throughout the entire combined system. This equilibrium temperature is an intensive property of the combined system.
Conclusion: Temperature's Intensive Nature is Paramount
In conclusion, temperature is undeniably an intensive property. Its value remains independent of the amount of matter in the system. This understanding is fundamental to various thermodynamic calculations, analyses, and applications. While heat transfer can cause changes in temperature, the temperature itself remains an inherent characteristic independent of the system's size or quantity. The distinction between temperature and heat, and understanding the concepts of extensive and intensive properties, is crucial for a solid grasp of thermodynamics. The concepts discussed here have applications far beyond the introductory level, and a full understanding of this fundamental principle allows for deeper exploration of the subject.
Latest Posts
Latest Posts
-
What Is Found In Both Dna And Rna
Mar 16, 2025
-
Rotational Symmetry Letters Of The Alphabet
Mar 16, 2025
-
What Is The Outermost Layer Of The Sun Called
Mar 16, 2025
-
What Are The Common Factors Of 25
Mar 16, 2025
-
Square Root Of 125 In Simplest Radical Form
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Is Temperature An Extensive Or Intensive Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
