Is Surface Tension Cohesion Or Adhesion
Juapaving
Mar 24, 2025 · 6 min read
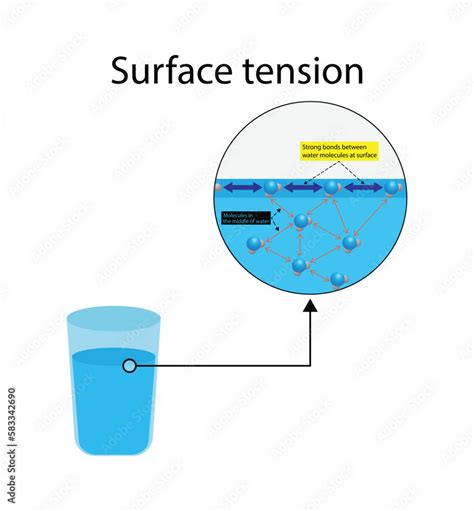
Table of Contents
Is Surface Tension Cohesion or Adhesion? Understanding Intermolecular Forces
Surface tension, that captivating phenomenon where a liquid's surface behaves like a stretched elastic membrane, is a fascinating display of intermolecular forces. But is it primarily driven by cohesion or adhesion? The answer, as with many things in science, is nuanced and depends on the context. While cohesion plays a crucial role, adhesion also significantly impacts the overall surface tension observed. Let's delve deep into the intricacies of these forces and how they contribute to this intriguing property of liquids.
Cohesion: The Internal Bonding of Liquids
Cohesion refers to the attractive forces between molecules of the same substance. In liquids, these forces are responsible for holding the molecules together, giving the liquid its characteristic bulk properties. These cohesive forces are typically van der Waals forces, including London dispersion forces, dipole-dipole interactions, and hydrogen bonding. The strength of these forces varies significantly depending on the type of molecule.
Strong Cohesive Forces: The Case of Water
Water, a quintessential example, exhibits exceptionally strong cohesive forces due to the presence of hydrogen bonding. Each water molecule can form up to four hydrogen bonds with neighboring molecules, creating a remarkably strong and interconnected network. This robust network is responsible for water's high surface tension, its relatively high boiling point, and its unique properties as a solvent.
Weaker Cohesive Forces: Impact on Surface Tension
Liquids with weaker cohesive forces, such as hydrocarbons, exhibit lower surface tension. The weaker intermolecular interactions mean the molecules are less strongly attracted to each other, resulting in a less "tightly packed" surface and a lower resistance to deformation.
Adhesion: The Attraction to Other Substances
Adhesion, on the other hand, refers to the attractive forces between molecules of different substances. This force is what allows liquids to wet surfaces. When a liquid comes into contact with a solid surface, the adhesive forces between the liquid molecules and the surface molecules come into play. If the adhesive forces are stronger than the cohesive forces within the liquid, the liquid will spread out over the surface, exhibiting a high degree of wetting.
The Role of Surface Energy
Adhesion is intimately related to the concept of surface energy. Molecules at the surface of a liquid experience an imbalance of forces. They are attracted to the molecules within the bulk liquid, but they lack neighboring molecules on one side. This imbalance results in a net inward force, causing the surface to contract and minimize its area – a key characteristic of surface tension. The stronger the adhesive forces between the liquid and the surface, the lower the surface energy of the liquid.
Wetting and Contact Angle
The balance between cohesive and adhesive forces determines the contact angle of a liquid on a surface. A low contact angle (close to 0°) indicates strong adhesion and good wetting, as the liquid spreads out over the surface. A high contact angle (close to 180°) indicates weak adhesion and poor wetting, with the liquid forming beads on the surface. This is crucial in various applications, from painting to printing to material science.
The Interplay of Cohesion and Adhesion in Surface Tension
Surface tension isn't solely determined by cohesion or adhesion; it's a complex interplay between the two. While cohesion holds the liquid together, adhesion influences how the liquid interacts with the surrounding environment. The net effect determines the overall surface tension observed.
Case Study: Water on Different Surfaces
Consider water on different surfaces:
-
On a clean glass surface: Water exhibits strong adhesion due to the interaction between the polar water molecules and the polar silica groups in the glass. The adhesive forces exceed the cohesive forces, leading to a low contact angle and excellent wetting. The surface tension is still present, but the liquid spreads to minimize the surface area in contact with air.
-
On a waxed surface: The hydrophobic nature of wax prevents strong adhesion between water molecules and the surface. The cohesive forces within the water dominate, resulting in a high contact angle and poor wetting (water beads up). The high surface tension manifests as a spherical droplet minimizing surface area.
-
On a Teflon surface: Teflon, a highly non-polar material, exhibits extremely weak adhesion to water. Water forms almost perfect spheres (high contact angle), demonstrating the dominance of cohesive forces. The surface tension is incredibly high for the droplet size.
Measuring Surface Tension: Techniques and Significance
Several methods exist for measuring surface tension, each utilizing the principles of interfacial forces. These techniques help us quantify the strength of surface tension and investigate the influence of various factors, such as temperature and the presence of surfactants. Key techniques include:
-
Du Noüy ring method: A platinum ring is carefully pulled away from the liquid surface, and the force required is measured. This force is directly related to the surface tension.
-
Wilhelmy plate method: A thin plate is partially submerged in the liquid, and the force required to maintain its position is measured. Similar to the Du Noüy ring method, it provides a quantitative measure of surface tension.
-
Pendant drop method: The shape of a liquid drop hanging from a capillary is analyzed, and the surface tension can be determined from the drop's dimensions.
Understanding surface tension is critical across many scientific disciplines and industrial applications. From the design of microfluidic devices to the development of new coatings and detergents, a firm grasp of the underlying principles is essential.
The Importance of Surface Tension in Everyday Life and Industry
Surface tension isn't just a fascinating laboratory phenomenon; it plays a crucial role in numerous everyday occurrences and industrial processes. Here are a few examples:
-
Capillary action: This is the ability of a liquid to flow in narrow spaces without the assistance of, or even in opposition to, external forces like gravity. It's essential for plant water uptake, and it's utilized in various technologies, such as chromatography and wicking materials. The balance between cohesive and adhesive forces determines the height a liquid will rise in a capillary tube.
-
Detergents and surfactants: These substances reduce surface tension by disrupting the cohesive forces between water molecules, allowing the water to penetrate and wet surfaces more effectively. This is crucial for cleaning, as it allows the detergent to remove dirt and grease.
-
Insect locomotion on water: Some insects can walk on water due to the high surface tension of water. Their weight is insufficient to break the surface.
-
Foam formation: Foam is created by trapping air bubbles within a liquid. Surface tension stabilizes the bubbles and creates the characteristic structure of foam.
-
Microfluidics: Surface tension plays a crucial role in the manipulation of fluids in microfluidic devices. The small scales involved mean surface forces dominate, allowing for precise control of fluid flow.
-
Coatings and Paints: The surface tension of coatings and paints influences their spreading and adhesion to surfaces. Controlling surface tension ensures uniform application and a high-quality finish.
Conclusion: A Balancing Act
Surface tension is a powerful testament to the intricate interplay of intermolecular forces. It's not solely driven by cohesion or adhesion; rather, it's a delicate balance between these two forces, influenced by the specific properties of the liquid and the interacting surface. Understanding this balance is paramount in various fields, allowing us to harness the power of surface tension for practical applications while appreciating its fundamental scientific significance. The study of surface tension continues to reveal new insights and possibilities, underscoring its enduring importance in science and technology.
Latest Posts
Latest Posts
-
Line Of Best Fit Equation Calculator
Mar 26, 2025
-
What Is The Half Of 24
Mar 26, 2025
-
Is The Number 23 Prime Or Composite
Mar 26, 2025
-
Is Square Root Of 15 A Rational Number
Mar 26, 2025
-
Which Of These Is A Polysaccharide
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Surface Tension Cohesion Or Adhesion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
