Is Sulfuric Acid A Strong Electrolyte
Juapaving
Mar 24, 2025 · 5 min read
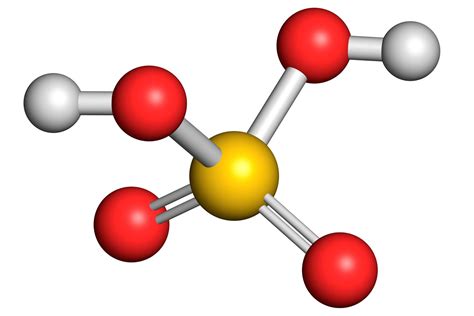
Table of Contents
Is Sulfuric Acid a Strong Electrolyte? A Deep Dive into its Properties and Behavior
Sulfuric acid (H₂SO₄), a highly corrosive strong mineral acid, is ubiquitous in various industrial processes and chemical reactions. One of its key properties that dictates its behavior and applications is its classification as a strong electrolyte. But what does that actually mean, and why is sulfuric acid considered such? This article delves into the intricacies of sulfuric acid's electrolytic nature, exploring its ionization, conductivity, and applications stemming from this crucial property.
Understanding Electrolytes and Their Strength
Before diving into the specifics of sulfuric acid, let's establish a foundation of understanding regarding electrolytes. An electrolyte is a substance that, when dissolved in a suitable solvent (like water), produces a solution that can conduct electricity. This conductivity arises from the presence of freely moving ions—charged particles—within the solution. These ions are formed when the electrolyte dissociates or ionizes into its constituent cations (positively charged ions) and anions (negatively charged ions).
The strength of an electrolyte is determined by the extent of its ionization or dissociation in solution. Strong electrolytes dissociate almost completely into ions, while weak electrolytes only partially dissociate. This difference significantly impacts the conductivity of the solution; strong electrolyte solutions are far better conductors of electricity than weak electrolyte solutions.
Strong Electrolytes vs. Weak Electrolytes: A Crucial Distinction
The distinction between strong and weak electrolytes is paramount in understanding their behavior and applications. Consider these differences:
-
Strong Electrolytes: These completely or almost completely dissociate into ions when dissolved. Examples include strong acids (like sulfuric acid, hydrochloric acid, nitric acid), strong bases (like sodium hydroxide, potassium hydroxide), and most soluble salts. Their solutions exhibit high electrical conductivity.
-
Weak Electrolytes: These only partially dissociate into ions, maintaining a significant equilibrium between the undissociated molecules and the ions. Examples include weak acids (like acetic acid, carbonic acid), weak bases (like ammonia), and some slightly soluble salts. Their solutions exhibit low electrical conductivity.
Sulfuric Acid's Complete Ionization: The Hallmark of a Strong Electrolyte
Sulfuric acid's status as a strong electrolyte stems from its near-complete ionization in aqueous solutions. The first ionization step is essentially complete:
H₂SO₄(aq) → H⁺(aq) + HSO₄⁻(aq)
This means that almost every molecule of sulfuric acid donates one proton (H⁺) to a water molecule, forming a hydronium ion (H₃O⁺) and a bisulfate ion (HSO₄⁻). The concentration of undissociated H₂SO₄ is negligible.
However, the second ionization step is not as complete:
HSO₄⁻(aq) ⇌ H⁺(aq) + SO₄²⁻(aq)
The bisulfate ion (HSO₄⁻) is a weak acid, meaning it only partially ionizes to yield more protons and sulfate ions (SO₄²⁻). While the first ionization is essentially quantitative, the second ionization is an equilibrium reaction, with a significant amount of HSO₄⁻ remaining in solution.
The Significance of the First Ionization
Despite the incomplete second ionization, sulfuric acid's first ionization step is the defining factor in classifying it as a strong electrolyte. The near-complete dissociation in the first step generates a high concentration of ions, leading to high electrical conductivity. This characteristic is crucial for many of its industrial applications.
Applications Leveraging Sulfuric Acid's Electrolytic Properties
Sulfuric acid's strong electrolytic nature underpins numerous applications across various industries:
-
Electroplating: The high ion concentration in sulfuric acid solutions makes it an excellent electrolyte in electroplating processes. It facilitates the deposition of metals onto surfaces, creating protective or decorative coatings.
-
Batteries: Lead-acid batteries, a common type of rechargeable battery, utilize sulfuric acid as the electrolyte. The acid's conductivity allows the flow of ions between the electrodes, enabling the battery's function.
-
Electrolytic Refining: Sulfuric acid's strong electrolytic properties are crucial in electrolytic refining processes, purifying metals like copper by using an electric current to remove impurities.
-
Industrial Chemical Processes: Numerous industrial chemical processes rely on sulfuric acid's ability to conduct electricity. This includes processes involving electrolysis or electrochemical reactions.
-
pH Control: In various applications, precise pH control is essential. Sulfuric acid, due to its complete first ionization, effectively lowers the pH of solutions, providing a means of controlling acidity.
Factors Affecting Sulfuric Acid's Conductivity
While sulfuric acid is a strong electrolyte, certain factors can influence its conductivity:
-
Concentration: The conductivity of sulfuric acid solutions increases with concentration up to a certain point, after which it may decrease slightly due to interionic interactions. Highly concentrated sulfuric acid exhibits lower conductivity due to increased viscosity and reduced ion mobility.
-
Temperature: Like most electrolytes, sulfuric acid's conductivity increases with temperature. Increased temperature boosts ion mobility, enhancing conductivity.
-
Presence of Impurities: The presence of impurities can affect conductivity. Impurities may interfere with ion mobility or introduce competing reactions, altering the overall conductivity.
Comparing Sulfuric Acid to Other Strong Electrolytes
To further solidify sulfuric acid's classification as a strong electrolyte, let's briefly compare it to other commonly known strong electrolytes:
-
Hydrochloric Acid (HCl): HCl is another strong acid that undergoes complete ionization in aqueous solutions. Its conductivity is comparable to that of sulfuric acid at similar concentrations.
-
Nitric Acid (HNO₃): Similar to HCl, nitric acid also completely ionizes, exhibiting strong electrolytic behavior.
-
Sodium Hydroxide (NaOH): A strong base, NaOH also dissociates completely in water, leading to high conductivity.
While these electrolytes share the characteristic of strong electrolytic behavior, the specific applications and properties vary depending on their individual chemical characteristics beyond just their electrolytic strength.
Conclusion: The Undeniable Strength of Sulfuric Acid's Electrolytic Nature
In conclusion, sulfuric acid unequivocally qualifies as a strong electrolyte. Its near-complete first ionization, resulting in a high concentration of ions in solution, leads to its exceptional electrical conductivity. This property is fundamental to its widespread use across a vast spectrum of industrial applications, from electroplating and battery production to electrolytic refining and pH control. Understanding the intricacies of sulfuric acid's electrolytic behavior is crucial for appreciating its significance in various chemical and industrial processes. The incomplete second ionization, while noteworthy, does not diminish its classification as a strong electrolyte due to the dominant influence of its complete first ionization.
Latest Posts
Latest Posts
-
What Is The Lcm Of 2 And 8
Mar 28, 2025
-
What Is The Name Of Mg No3 2
Mar 28, 2025
-
Which Of The Following Is True About Hiv
Mar 28, 2025
-
In Which Situation Is The Distance Traveled Proportional To Time
Mar 28, 2025
-
Which Group Of Nonmetals Is The Most Reactive
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Sulfuric Acid A Strong Electrolyte . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
