Is Sugar Dissolves In Water A Physical Or Chemical Change
Juapaving
Mar 23, 2025 · 5 min read
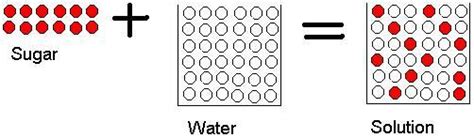
Table of Contents
Is Dissolving Sugar in Water a Physical or Chemical Change? A Deep Dive
The question of whether dissolving sugar in water is a physical or chemical change is a classic introductory chemistry conundrum. While seemingly simple, it delves into the fundamental concepts of matter, its states, and the transformations it undergoes. Understanding the distinction between physical and chemical changes is crucial for comprehending various scientific phenomena and processes. This article will explore the dissolution of sugar in water, examining the evidence, debunking common misconceptions, and clarifying why it's categorized as a physical change.
Understanding Physical and Chemical Changes
Before we dive into the specifics of sugar and water, let's establish a clear understanding of the difference between physical and chemical changes.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but does not change its chemical composition. The molecules remain the same; only their arrangement or state of matter might change. Examples include:
- Changes in state: Melting ice (solid to liquid), boiling water (liquid to gas), freezing water (liquid to solid). The water molecules remain H₂O throughout.
- Dissolving: Salt dissolving in water, or sugar dissolving in water (as we'll examine in detail). The salt or sugar molecules are dispersed, but their chemical structure is unchanged.
- Crushing: Breaking a rock into smaller pieces. The chemical composition of the rock remains the same.
- Cutting: Cutting paper or slicing an apple. The chemical makeup of the paper or apple doesn't change.
Chemical Changes: Breaking and Making Bonds
A chemical change, also known as a chemical reaction, involves the formation of new substances with different chemical properties than the starting materials. This involves the breaking and forming of chemical bonds, rearranging atoms into new molecules. Examples include:
- Burning: Burning wood or paper. The cellulose in the wood or paper reacts with oxygen to produce carbon dioxide, water, and ash.
- Rusting: Iron reacting with oxygen and water to form iron oxide (rust).
- Cooking: Cooking an egg involves irreversible chemical changes to the proteins within the egg.
- Digestion: The breakdown of food in our bodies involves numerous chemical reactions.
Analyzing the Dissolution of Sugar in Water
Now, let's focus on the specific case of dissolving sugar (sucrose, C₁₂H₂₂O₁₁) in water (H₂O). When you stir sugar into water, it appears to disappear, forming a clear, sweet solution. However, does this mean a chemical change has occurred?
Evidence for a Physical Change:
Several key observations support the classification of dissolving sugar in water as a physical change:
- No new substance is formed: The sugar molecules (sucrose) and water molecules (H₂O) remain the same. No new chemical bonds are formed, and no existing bonds are broken. The sweet taste comes from the presence of unaltered sugar molecules dispersed in the water.
- Reversible process: The sugar can be easily recovered by evaporating the water. This would leave behind the original crystalline sugar, demonstrating that the sugar's chemical structure has not been altered. This reversibility is a hallmark of physical changes.
- No energy change (significant): While there might be a slight temperature change due to the dissolution process (the solution might become slightly cooler), this is minimal and doesn't represent a significant energy transformation associated with chemical reactions. Chemical reactions often involve noticeable heat release (exothermic) or absorption (endothermic).
- Sugar molecules remain intact: Advanced techniques like spectroscopy can confirm that the molecular structure of sucrose remains intact in the solution. The sugar molecules are simply surrounded and separated by water molecules.
Addressing Common Misconceptions
Some individuals might mistakenly believe that dissolving sugar is a chemical change because:
- Appearance change: The sugar seems to disappear, creating a homogeneous mixture. This change in appearance is deceptive. Physical changes frequently involve alterations in appearance without fundamentally changing the underlying substance.
- Sweetness: The sweet taste might lead to the misconception that a new substance is formed. The sweetness originates from the sugar molecules, which are still present, albeit dispersed in the water.
The Role of Water Molecules
The key to understanding why dissolving sugar is a physical change lies in the interaction between sugar and water molecules. Water molecules are polar, meaning they have a slightly positive end and a slightly negative end. The sugar molecules are also polar, and this polarity allows them to interact with the water molecules through a process called solvation.
In solvation, the water molecules surround the sugar molecules, effectively separating them and allowing them to disperse evenly throughout the water. This process doesn't involve the breaking or forming of chemical bonds. The water molecules simply form weak interactions (hydrogen bonds) with the sugar molecules, keeping them apart and dissolved.
Beyond Sugar and Water: Generalizing the Concept
The principle of dissolving a substance in water (or another solvent) being generally a physical change can be applied to other substances. Many salts, for instance, dissolve in water through a similar solvation process. However, there are exceptions. Some substances undergo chemical reactions when dissolved in water, such as certain metal oxides or acids which react with water, leading to new chemical species.
Applications and Significance
The understanding of physical changes, such as dissolving sugar in water, is crucial in various fields:
- Food science: Dissolving sugar and other ingredients is a fundamental process in many food preparations.
- Pharmacology: Dissolving medications is essential for their absorption and distribution in the body.
- Chemistry: The concept of solubility and dissolution is central to numerous chemical processes and analyses.
- Environmental science: Understanding how substances dissolve in water is crucial for studying water pollution and remediation.
Conclusion: Sugar Dissolving – A Definitive Physical Change
In conclusion, dissolving sugar in water is undoubtedly a physical change. No new substance is formed; the sugar molecules remain intact. The process is reversible, and there's no significant energy change. The apparent disappearance of the sugar is merely a consequence of its molecules being dispersed and surrounded by water molecules through solvation. While seemingly a simple observation, this distinction highlights the fundamental differences between physical and chemical transformations, a core concept in chemistry and related scientific disciplines. Understanding this difference is key to comprehending a vast array of natural and engineered processes.
Latest Posts
Latest Posts
-
How Many Feet Is 37 Inches
Mar 25, 2025
-
Lcm Of 12 15 And 9
Mar 25, 2025
-
Whats The Square Root Of 10
Mar 25, 2025
-
Whats The Prime Factorization Of 40
Mar 25, 2025
-
What Is The Atomic Symbol For Potassium
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Is Sugar Dissolves In Water A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
