Is Salt A Compound Or Mixture
Juapaving
Mar 07, 2025 · 6 min read
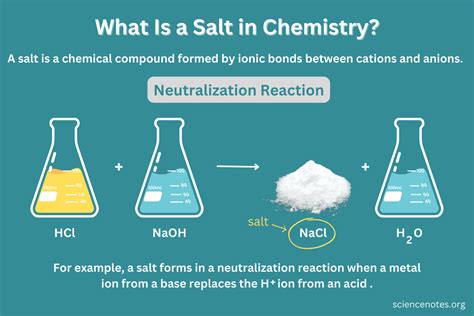
Table of Contents
Is Salt a Compound or a Mixture? A Deep Dive into Chemical Composition
The seemingly simple question, "Is salt a compound or a mixture?", opens a fascinating window into the world of chemistry. While the answer might seem straightforward at first glance, a deeper understanding requires exploring the fundamental differences between compounds and mixtures and examining the properties of salt itself. This article will delve into the intricacies of chemical composition, clarifying the nature of salt and providing a comprehensive understanding of its classification.
Understanding Compounds and Mixtures
Before classifying salt, let's establish a clear understanding of compounds and mixtures. These two categories represent distinct ways in which substances can combine.
Compounds: The Chemical Bond
A compound is a substance formed when two or more chemical elements are chemically bonded together. This bonding involves a fundamental rearrangement of the constituent atoms, resulting in a new substance with properties different from its constituent elements. The key characteristic of a compound is that its composition is fixed and definite, meaning the ratio of elements is always the same. Compounds can only be separated into their constituent elements through chemical reactions, not by simple physical methods like filtration or evaporation. The bonds holding the atoms together can be ionic (like in salt) or covalent (like in water).
Mixtures: A Physical Combination
A mixture, on the other hand, is a combination of two or more substances that are not chemically bonded. The substances retain their individual chemical properties, and their proportions can vary. Mixtures can be separated into their components through physical means, such as filtration, distillation, or evaporation. Examples include air (a mixture of gases), saltwater (a mixture of salt and water), and sand (a mixture of different minerals).
The Case of Salt: Sodium Chloride (NaCl)
Common table salt, also known chemically as sodium chloride (NaCl), is an ionic compound. It's formed through the ionic bonding of sodium (Na), a highly reactive metal, and chlorine (Cl), a highly reactive nonmetal. This bonding involves the transfer of an electron from the sodium atom to the chlorine atom, creating positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). The electrostatic attraction between these oppositely charged ions forms the strong ionic bond characteristic of salt crystals.
Evidence Supporting Salt as a Compound
Several pieces of evidence strongly support the classification of salt as a compound:
-
Fixed Composition: Pure sodium chloride always contains the same ratio of sodium and chlorine atoms: one sodium atom for every chlorine atom. This fixed ratio is a hallmark of compounds. You can't have a salt with varying proportions of sodium and chlorine and still call it pure sodium chloride.
-
Distinct Properties: The properties of sodium chloride are significantly different from those of its constituent elements. Sodium is a highly reactive metal that violently reacts with water, while chlorine is a toxic, greenish-yellow gas. Sodium chloride, on the other hand, is a crystalline solid, readily soluble in water, and relatively unreactive. This difference in properties is indicative of a chemical change, not a simple physical mixing.
-
Chemical Decomposition: Salt can be decomposed into its constituent elements only through chemical means, such as electrolysis. This process requires an external energy source to break the strong ionic bonds holding the sodium and chlorine ions together. Simple physical methods cannot separate sodium and chlorine from salt.
-
Crystal Structure: The crystalline structure of salt is a direct result of the ordered arrangement of sodium and chloride ions in a three-dimensional lattice. This regular, repeating pattern wouldn't exist in a simple mixture.
Addressing Common Misconceptions
Some might argue that salt dissolved in water is a mixture. This is partially true, but it's crucial to understand the distinction. The dissolved salt in water is a homogeneous mixture, meaning the salt is uniformly dispersed throughout the water. However, the salt itself remains a compound; it hasn't changed its chemical composition. The dissolution process is a physical change, separating the individual ions, but the ionic bonds remain intact until further chemical reaction. You can recover the original salt by evaporating the water.
Similarly, table salt often contains additives like iodine or anticaking agents. In such cases, the table salt is technically a mixture of sodium chloride and these additives. However, the sodium chloride component remains a compound. Therefore, the overall classification depends on the context: pure sodium chloride is a compound; table salt, with its additives, is a mixture.
Salt in Different Forms: Still a Compound
Regardless of the form – whether it's a crystalline solid, a solution, or a mixture with additives – the chemical nature of sodium chloride remains unchanged. The fundamental building block of salt – the ionic bond between sodium and chlorine – persists, confirming its status as a compound. The presence of other substances might alter its physical properties or create a mixture, but it doesn't change the underlying chemical composition of sodium chloride itself.
The Importance of Understanding Chemical Classification
Correctly identifying a substance as a compound or a mixture is crucial for several reasons:
-
Predicting Properties: Understanding the chemical composition allows scientists to predict the properties of a substance. Knowing that salt is an ionic compound allows us to predict its solubility in water, its melting point, and its electrical conductivity when dissolved.
-
Chemical Reactions: The reactivity of a substance is directly linked to its chemical composition. Understanding that salt is a compound allows us to predict how it will react with other substances.
-
Applications and Uses: The properties of a substance dictate its applications. The unique properties of sodium chloride, stemming from its compound nature, lead to its use as a preservative, flavor enhancer, and in many industrial processes.
-
Environmental Impact: Knowing the chemical composition of a substance helps assess its potential environmental impact. The behavior of sodium chloride in the environment, its solubility and its interaction with other chemicals, are all linked to its nature as a compound.
Conclusion: Salt – A Fundamental Compound
In conclusion, while salt can be part of mixtures (like saltwater or iodized salt), pure sodium chloride is unequivocally a compound. Its fixed composition, distinct properties, the requirement of chemical means for decomposition, and its crystalline structure all firmly establish its status as a chemically bonded substance. Understanding this fundamental classification is crucial for comprehending the behavior of salt and its diverse applications in various fields. The seemingly simple question of whether salt is a compound or a mixture leads to a deeper appreciation for the fundamental principles of chemistry and the intricate nature of chemical bonding.
Latest Posts
Latest Posts
-
Which Of The Following Is Not An Si Base Unit
Mar 09, 2025
-
What Country Is Called The Land Of The Rising Sun
Mar 09, 2025
-
What Are All The Factors For 40
Mar 09, 2025
-
Which Is A Characteristic Of A Mixture
Mar 09, 2025
-
What Is The Highest Common Factor Of 28 And 42
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about Is Salt A Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
