Is N More Electronegative Than C
Juapaving
Mar 28, 2025 · 6 min read
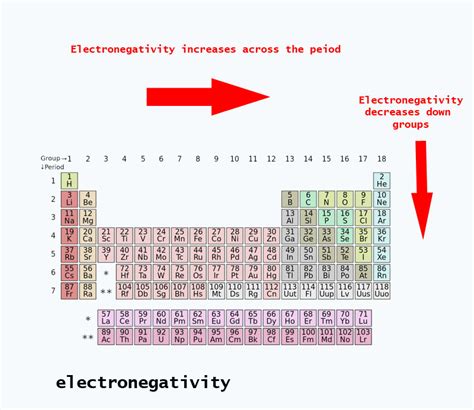
Table of Contents
Is N More Electronegative Than C? A Deep Dive into Electronegativity
Electronegativity, a fundamental concept in chemistry, dictates the tendency of an atom to attract a shared pair of electrons in a covalent bond. Understanding electronegativity differences is crucial for predicting molecular polarity, bond strength, and reactivity. This article will delve into the comparison of nitrogen (N) and carbon (C) electronegativity, exploring the underlying reasons for the difference and its implications in various chemical contexts. We'll explore the periodic trends that govern electronegativity, examining the atomic structure of both nitrogen and carbon to understand why nitrogen exhibits higher electronegativity.
Understanding Electronegativity: A Brief Overview
Electronegativity isn't a directly measurable quantity like mass or charge. Instead, it's a relative property, typically represented using the Pauling scale, where fluorine (F) is assigned the highest value of 4.0. Elements with higher electronegativity values attract electrons more strongly than those with lower values. This difference in electronegativity between atoms in a bond creates a dipole moment, leading to polar covalent bonds. The greater the difference in electronegativity, the more polar the bond becomes.
Factors Affecting Electronegativity
Several factors contribute to an element's electronegativity:
- Nuclear Charge: A higher nuclear charge means a stronger attraction for electrons.
- Atomic Radius: Smaller atoms hold electrons closer to the nucleus, increasing electronegativity.
- Shielding Effect: Inner electrons shield outer electrons from the full nuclear charge, reducing electronegativity.
- Effective Nuclear Charge: This is the net positive charge experienced by valence electrons, considering both nuclear charge and shielding. It's a key determinant of electronegativity.
Comparing Nitrogen (N) and Carbon (C) Electronegativity
The Pauling scale assigns nitrogen an electronegativity of 3.04 and carbon an electronegativity of 2.55. This clearly indicates that nitrogen is more electronegative than carbon. But why? Let's explore the reasons behind this difference:
Atomic Structure and Electronegativity
Both nitrogen and carbon are located in the second period of the periodic table, but in different groups. Nitrogen is in group 15 (pnictogens), while carbon is in group 14 (carbon group). This difference in group placement significantly impacts their electronegativity:
-
Nitrogen (N): Nitrogen has seven electrons: two in the first shell and five in the second (valence) shell. It needs three more electrons to achieve a stable octet configuration. This strong desire for three additional electrons contributes to its high electronegativity. Its smaller atomic radius compared to carbon also plays a significant role.
-
Carbon (C): Carbon has six electrons: two in the first shell and four in the valence shell. It requires four more electrons to complete its octet. While still needing electrons to achieve stability, its slightly larger atomic radius and slightly lower effective nuclear charge compared to nitrogen result in lower electronegativity.
Periodic Trends and Electronegativity
The periodic table provides valuable insights into electronegativity trends. Electronegativity generally:
- Increases across a period: Moving from left to right, the nuclear charge increases while the atomic radius decreases, resulting in a stronger pull on electrons. This is why nitrogen is more electronegative than carbon, as they are in the same period but nitrogen is further to the right.
- Decreases down a group: Moving down a group, the atomic radius increases significantly, shielding the outer electrons from the nuclear charge and weakening the attraction.
Nitrogen and carbon both reside in the second period, therefore the trend across the period is the dominant factor in this comparison.
Implications of the Electronegativity Difference
The difference in electronegativity between nitrogen and carbon has profound consequences for their chemical behavior and the properties of molecules containing both elements:
Polarity of Bonds
In a C-N bond, the nitrogen atom attracts the shared electrons more strongly, leading to a polar covalent bond. The nitrogen atom carries a partial negative charge (δ-), while the carbon atom carries a partial positive charge (δ+). This polarity influences the overall polarity of molecules, impacting their physical and chemical properties. For example, molecules with multiple polar C-N bonds may be soluble in polar solvents like water.
Bond Strength
While electronegativity primarily affects bond polarity, it also indirectly influences bond strength. The stronger attraction of nitrogen for shared electrons can contribute to a slightly stronger C-N bond compared to a C-C bond. However, other factors, such as bond order and hybridization, play a more significant role in determining absolute bond strength.
Reactivity
The difference in electronegativity between nitrogen and carbon directly affects their reactivity. Nitrogen's higher electronegativity makes it less likely to donate electrons and more likely to accept them, affecting its reactivity in different chemical environments. For instance, nitrogen often participates in reactions involving coordinate covalent bonds, where it donates a lone pair of electrons. In contrast, carbon's lower electronegativity allows it to participate more readily in reactions involving both electron donation and acceptance.
Examples in Organic Chemistry
Many organic compounds contain both carbon and nitrogen atoms. The electronegativity difference between these atoms significantly influences the properties and reactivity of these molecules. Let's consider some examples:
Nitriles (R-CN)
Nitriles contain a C≡N triple bond. The high electronegativity of nitrogen polarizes this bond, giving the nitrogen atom a partial negative charge. This polarity makes nitriles relatively polar molecules, affecting their solubility and reactivity.
Amines (R-NH2, R2NH, R3N)
Amines contain a C-N single bond. The polarity of the C-N bond influences the basicity of amines. The nitrogen atom's lone pair of electrons is more available for protonation due to the partially negative charge, resulting in the basic properties of amines.
Amides (R-CONH2)
Amides contain a C-N bond within a carbonyl group. The electronegativity difference between carbon and nitrogen combined with the electron-withdrawing nature of the carbonyl group impacts the reactivity and properties of amides. This leads to their relatively low basicity compared to amines.
Advanced Concepts and Applications
Understanding the electronegativity difference between nitrogen and carbon extends to advanced concepts in chemistry and material science:
Organic Synthesis and Drug Design
The polar nature of C-N bonds and the reactivity of nitrogen-containing functional groups are crucial aspects of organic synthesis and drug design. Researchers carefully consider electronegativity effects when designing new molecules with specific properties.
Material Science and Nanotechnology
Nitrogen's incorporation into materials often modifies their electronic and optical properties. The electronegativity difference between nitrogen and carbon plays a role in the design and functionality of advanced materials such as carbon nitride polymers.
Computational Chemistry
Computational chemistry methods, such as density functional theory (DFT), can accurately predict and quantify electronegativity differences, helping to model and understand the properties of molecules containing both nitrogen and carbon atoms.
Conclusion
In summary, nitrogen is demonstrably more electronegative than carbon due to its higher effective nuclear charge and smaller atomic radius. This difference in electronegativity significantly influences the polarity of C-N bonds, the reactivity of nitrogen-containing functional groups, and the overall properties of molecules containing both elements. Understanding this fundamental concept is crucial for comprehending a wide range of chemical phenomena and for applications in various fields, including organic chemistry, material science, and drug design. The relatively simple comparison of nitrogen and carbon electronegativity reveals the powerful impact that even small differences in electronegativity can have on the properties and behavior of molecules. Further exploration into the interplay of electronegativity, atomic structure, and periodic trends enhances the understanding of chemical bonding and molecular properties.
Latest Posts
Latest Posts
-
How Many Vertices Do A Square Have
Mar 31, 2025
-
Lcm Of 3 9 And 12
Mar 31, 2025
-
How Many Feet In 95 Inches
Mar 31, 2025
-
350 Square Meters In Square Feet
Mar 31, 2025
-
Explain How Software Is Distinct From Hardware
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is N More Electronegative Than C . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
