Is Milk Of Magnesia A Base Or An Acid
Juapaving
Mar 19, 2025 · 5 min read
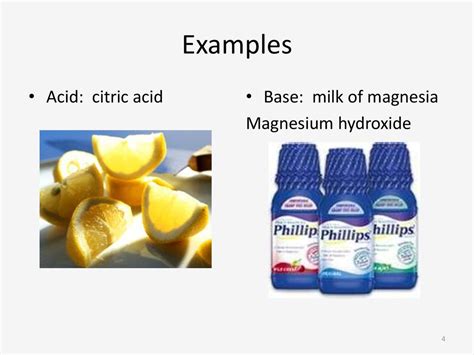
Table of Contents
- Is Milk Of Magnesia A Base Or An Acid
- Table of Contents
- Is Milk of Magnesia a Base or an Acid? Understanding pH and Antacids
- Understanding pH: The Acid-Base Spectrum
- The Chemical Composition of Milk of Magnesia
- How Magnesium Hydroxide Works
- Milk of Magnesia vs. Other Antacids
- The Importance of pH in Biological Systems
- Potential Side Effects and Precautions
- Conclusion: Milk of Magnesia – A Basic Remedy
- Latest Posts
- Latest Posts
- Related Post
Is Milk of Magnesia a Base or an Acid? Understanding pH and Antacids
Milk of magnesia, a common household remedy, is frequently used for treating constipation and heartburn. But have you ever wondered about its chemical nature? Is it an acid or a base? The answer, simply put, is that milk of magnesia is a base. Understanding why requires a dive into the world of pH, acids, and bases. This comprehensive article will explore the chemical composition of milk of magnesia, its effects on the body, and its classification within the pH scale.
Understanding pH: The Acid-Base Spectrum
Before we delve into the specifics of milk of magnesia, let's establish a foundational understanding of the pH scale. The pH scale measures the acidity or basicity (alkalinity) of a solution. It ranges from 0 to 14, with:
- 0-6 considered acidic: Substances with a pH less than 7 donate protons (H+) in solution. Common examples include lemon juice, vinegar, and stomach acid.
- 7 considered neutral: Pure water has a pH of 7, meaning it neither donates nor accepts protons.
- 8-14 considered basic (alkaline): Substances with a pH greater than 7 accept protons (H+) in solution. Common examples include baking soda, ammonia, and, crucially for this discussion, milk of magnesia.
The pH scale is logarithmic, meaning each whole number change represents a tenfold difference in acidity or alkalinity. A pH of 3 is ten times more acidic than a pH of 4, and a hundred times more acidic than a pH of 5.
The Chemical Composition of Milk of Magnesia
Milk of magnesia, also known as magnesium hydroxide (Mg(OH)₂), is a suspension of magnesium hydroxide in water. This chemical compound is inherently basic. When dissolved in water, it undergoes dissociation, releasing magnesium ions (Mg²⁺) and hydroxide ions (OH⁻). These hydroxide ions are what contribute to the basic nature of the solution. The presence of these hydroxide ions is the key to understanding why milk of magnesia has a high pH, typically around 10.
How Magnesium Hydroxide Works
The hydroxide ions (OH⁻) in milk of magnesia are crucial to its antacid and laxative properties. Let's explore both:
1. Antacid Action: Stomach acid, primarily hydrochloric acid (HCl), is highly acidic (pH around 1-2). When milk of magnesia is ingested, the hydroxide ions neutralize the excess stomach acid through a neutralization reaction. This reaction produces water (H₂O) and magnesium chloride (MgCl₂), a salt, thereby reducing the acidity in the stomach and relieving heartburn symptoms. The chemical equation is as follows:
2HCl (aq) + Mg(OH)₂ (s) → MgCl₂ (aq) + 2H₂O (l)
2. Laxative Action: The magnesium ions (Mg²⁺) in milk of magnesia also play a role, particularly in its laxative effect. Magnesium ions draw water into the intestines, softening the stool and stimulating bowel movements. This osmotic effect helps relieve constipation.
Milk of Magnesia vs. Other Antacids
Compared to other antacids, milk of magnesia stands out due to its high pH and its dual action as both an antacid and a laxative. Many other antacids are also bases, but they may have different active ingredients and mechanisms of action. For example, some antacids contain calcium carbonate (CaCO₃) or aluminum hydroxide (Al(OH)₃), both of which also neutralize stomach acid but do not have the same laxative effect as magnesium hydroxide.
The choice of antacid depends on individual needs and preferences. Some individuals may find milk of magnesia effective for both heartburn and constipation, while others might prefer a different antacid with a gentler laxative effect or one focusing solely on acid neutralization.
The Importance of pH in Biological Systems
The pH of various bodily fluids is tightly regulated to maintain optimal health. Deviations from the normal pH range can have significant consequences. For instance, the stomach's highly acidic environment is essential for digesting food and killing harmful bacteria. However, excessive stomach acid can lead to heartburn and ulcers. The intestines, on the other hand, have a slightly alkaline environment.
Milk of Magnesia's ability to neutralize stomach acid highlights the importance of maintaining pH balance in the body. Its basic nature helps restore the proper pH balance in the stomach when there's an excess of acid. However, it's crucial to use milk of magnesia as directed, as excessive consumption can lead to side effects like diarrhea.
Potential Side Effects and Precautions
While generally safe when used as directed, milk of magnesia can cause side effects, particularly with overuse. These can include:
- Diarrhea: Due to its laxative effect, excessive consumption can lead to diarrhea.
- Dehydration: Prolonged diarrhea can lead to dehydration.
- Electrolyte imbalances: Overconsumption can disrupt electrolyte balance in the body.
- Interactions with other medications: Milk of magnesia can interact with certain medications, so it’s important to consult a doctor or pharmacist if you're taking other drugs.
It's crucial to always follow the dosage instructions provided on the label or as recommended by a healthcare professional. If you experience any adverse effects, stop using the product and consult your doctor immediately. Pregnant or breastfeeding women, as well as individuals with underlying health conditions, should also seek advice from a healthcare professional before using milk of magnesia.
Conclusion: Milk of Magnesia – A Basic Remedy
Milk of magnesia is undeniably a base, its chemical composition (magnesium hydroxide) and resulting pH (around 10) clearly indicating its alkaline nature. This basic nature is responsible for its effectiveness as both an antacid and a laxative. Its ability to neutralize stomach acid and stimulate bowel movements makes it a versatile remedy for common digestive issues. However, responsible usage is key. Always follow dosage instructions, be mindful of potential side effects, and consult with a healthcare professional if you have any concerns. Understanding the pH scale and the chemical properties of medications like milk of magnesia empowers us to make informed decisions about our health and well-being. Remember to always consult with a healthcare professional before starting any new medication or treatment plan. Self-treating can be risky and could lead to unexpected health complications.
Latest Posts
Latest Posts
-
What Is 63 Kilos In Pounds
Mar 20, 2025
-
How Many Minutes In 40 Hours
Mar 20, 2025
-
Are Skin Cells Haploid Or Diploid
Mar 20, 2025
-
Real Life Examples Of Gay Lussacs Law
Mar 20, 2025
-
Least Common Multiple Of 2 3 And 5
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Milk Of Magnesia A Base Or An Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
