Real Life Examples Of Gay Lussac's Law
Juapaving
Mar 20, 2025 · 7 min read
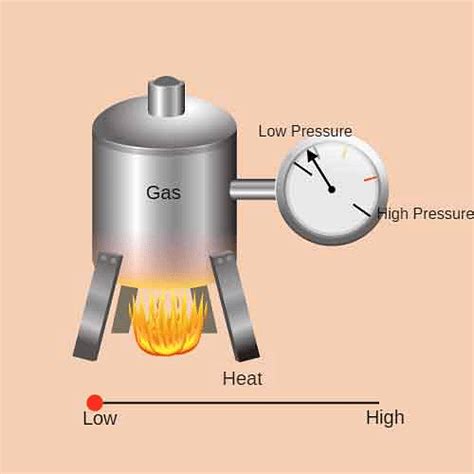
Table of Contents
Real-Life Examples of Gay-Lussac's Law: From Balloons to Pressure Cookers
Gay-Lussac's Law, also known as Amonton's Law, is a fundamental gas law that describes the relationship between the temperature and pressure of a gas when the volume is held constant. It states that the pressure of a given amount of gas held at constant volume is directly proportional to its absolute temperature. This means that as the temperature increases, the pressure also increases, and vice-versa. This seemingly simple law has profound implications and manifests itself in numerous everyday situations. Let's explore some compelling real-life examples that illustrate Gay-Lussac's Law in action.
Understanding the Basics: Pressure, Temperature, and Constant Volume
Before delving into specific examples, it's crucial to understand the core components of Gay-Lussac's Law:
-
Pressure (P): Measured in units like Pascals (Pa), atmospheres (atm), or pounds per square inch (psi), pressure represents the force exerted by gas molecules on the container walls.
-
Temperature (T): Measured in Kelvin (K), temperature reflects the average kinetic energy of gas molecules. It's crucial to use the Kelvin scale because it represents absolute temperature, where zero Kelvin represents the absence of all thermal energy.
-
Constant Volume (V): This is a key condition of Gay-Lussac's Law. The volume of the gas must remain unchanged for the law to accurately describe the relationship between pressure and temperature.
Mathematically, Gay-Lussac's Law is represented as:
P₁/T₁ = P₂/T₂
Where:
- P₁ is the initial pressure
- T₁ is the initial temperature
- P₂ is the final pressure
- T₂ is the final temperature
Real-Life Applications and Examples:
Now let's explore a variety of real-world examples where Gay-Lussac's Law plays a significant role:
1. Pressure Cookers: A Culinary Demonstration
Pressure cookers are a fantastic example of Gay-Lussac's Law in action. These appliances work by sealing food in a container and heating it. As the temperature inside the cooker increases, the pressure of the steam inside also increases significantly. This heightened pressure leads to higher cooking temperatures than are possible in a regular pot, resulting in faster cooking times and tenderized food.
How it relates to Gay-Lussac's Law: The volume inside the pressure cooker remains relatively constant. As the heat source increases the temperature (T₂ > T₁), the pressure within (P₂) correspondingly rises significantly higher than the initial pressure (P₁). The safety valve on the pressure cooker is crucial; it releases excess pressure to prevent dangerous over-pressurization.
2. Aerosol Cans: Controlled Release Under Pressure
Aerosol cans, from hairspray to whipped cream, leverage Gay-Lussac's Law for their functionality. These cans contain a pressurized gas that propels the product out. The pressure within the can is a result of the gas's temperature.
How it relates to Gay-Lussac's Law: The volume of the can remains largely constant. When the can is heated, even slightly by sunlight, the pressure inside increases. This increased pressure makes the dispensing of the product easier. Conversely, if the can is cooled, the pressure decreases, making dispensing more difficult. This is why storing aerosol cans in hot environments is generally discouraged.
3. Automobile Tires: Temperature and Pressure Fluctuations
The air pressure in your car tires is directly affected by temperature changes. On a hot summer day, the air inside your tires heats up, causing the pressure to increase. Conversely, on a cold winter day, the air cools down, leading to a decrease in tire pressure.
How it relates to Gay-Lussac's Law: The volume of the tire remains relatively constant, barring significant changes in shape or punctures. As the temperature increases (T₂ > T₁), the pressure of the air within the tire (P₂) rises accordingly. Regular tire pressure checks are essential, especially during seasonal temperature shifts, to ensure safe and optimal driving conditions.
4. Hot Air Balloons: Buoyancy Through Heated Air
Hot air balloons provide a visually striking demonstration of Gay-Lussac's Law. The balloon rises because the hot air inside is less dense than the surrounding cooler air. Heating the air increases its pressure, and the balloon's fabric expands to accommodate the increased volume, although not infinitely.
How it relates to Gay-Lussac's Law: While the volume is not strictly constant (it expands slightly), the relationship between temperature and pressure is still demonstrably relevant. Increasing the temperature (T₂) of the air within the balloon causes a substantial increase in pressure (P₂), which contributes to its lift by decreasing the air's overall density.
5. Weather Balloons: Measuring Atmospheric Pressure and Temperature
Weather balloons are regularly launched into the atmosphere to collect data on temperature, pressure, and humidity at different altitudes. The expansion and contraction of the balloon itself directly reflect changes in atmospheric pressure, which are in turn directly related to temperature changes as per Gay-Lussac's law.
How it relates to Gay-Lussac's Law: As the balloon ascends to higher altitudes, the atmospheric pressure decreases. Since the volume of the gas inside changes as well (it expands), this isn't a perfect example, however, the changes in temperature and pressure are still largely interdependent and can be modelled through ideal gas equations which encompass Gay-Lussac's Law as a sub-component.
6. Scuba Diving Tanks: Pressure and Temperature Considerations
Scuba diving tanks store compressed air at high pressure. The pressure inside the tank is influenced by the temperature of the surrounding environment. Changes in water temperature, especially in deep dives where temperature can differ significantly from surface, can affect the tank's pressure, impacting the dive's duration and safety.
How it relates to Gay-Lussac's Law: The volume of the tank remains constant. Temperature changes (T₁ vs T₂) directly impact the internal pressure (P₁ vs P₂). Divers need to be aware of these temperature-pressure relationships to adjust their dive plans accordingly and ensure adequate air supply throughout their dive.
7. Industrial Processes: Maintaining Pressure and Temperature
Numerous industrial processes rely on precise temperature and pressure control. These processes often involve gases, and understanding Gay-Lussac's Law is crucial for ensuring safety and efficiency. Chemical reactors, for instance, require careful monitoring of both temperature and pressure to optimize reactions and prevent explosions.
How it relates to Gay-Lussac's Law: In controlled environments, constant volume reactors maintain a fixed volume (V), and monitoring pressure (P) changes provides real-time feedback on temperature (T) changes. This constant monitoring is vital for reaction control and safety.
8. Liquefied Petroleum Gas (LPG) Tanks: Temperature-Pressure Relationship
LPG tanks, commonly used for cooking and heating, store propane or butane under high pressure. The pressure inside the tank varies with temperature. On hot days, the pressure increases; on cold days, it decreases. This is why it's important to handle LPG tanks with care, especially in extreme temperature conditions.
How it relates to Gay-Lussac's Law: The volume of the LPG tank remains constant. Increases in temperature (T₂) directly translate into increased pressure (P₂), emphasizing the need for safe handling and storage to prevent over-pressurization.
9. Bicycle Pumps: Building Pressure through Compression
When you use a bicycle pump, you're directly applying Gay-Lussac's Law. By compressing the air within the pump's cylinder (reducing its volume), you increase its temperature and thus, its pressure.
How it relates to Gay-Lussac's Law: While the volume is not strictly constant during the pumping action (it reduces and then the air is moved to a tire which is mostly constant volume), the process of compressing the air within the pump itself increases its temperature and pressure before transfer. The pressure increase is directly relatable to the increase in temperature (due to friction and compression) before transfer to the tyre.
Limitations of Gay-Lussac's Law
While Gay-Lussac's Law is a useful approximation, it has limitations:
-
Ideal Gas Assumption: The law assumes the gas behaves ideally, which means there are no intermolecular forces and the gas molecules occupy negligible volume. This assumption is only accurate at relatively low pressures and high temperatures. At higher pressures and lower temperatures, real gases deviate from ideal behavior.
-
Constant Volume Requirement: The law only applies when the volume of the gas is held constant. If the volume changes, then other gas laws, such as the combined gas law, must be considered.
Conclusion
Gay-Lussac's Law, despite its seemingly simple premise, underpins numerous aspects of our daily lives. From cooking with pressure cookers to driving on a hot day, the relationship between temperature and pressure of gases is constantly at play. Understanding this law offers valuable insight into various phenomena and highlights the importance of considering temperature fluctuations when dealing with pressurized systems and gases. Remember that while the law provides a good approximation, always consider the limitations and potential deviations from ideal gas behavior in real-world applications.
Latest Posts
Latest Posts
-
What Is The Smallest Unit That Makes Up Matter
Mar 20, 2025
-
The Time Rate Of Doing Work Is Called
Mar 20, 2025
-
What Is The Square Root Of 74
Mar 20, 2025
-
What Is The Purpose Of The Petals
Mar 20, 2025
-
What Is The Multiples Of 8
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Real Life Examples Of Gay Lussac's Law . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
