Is Melting Butter A Physical Or Chemical Change
Juapaving
Mar 17, 2025 · 5 min read
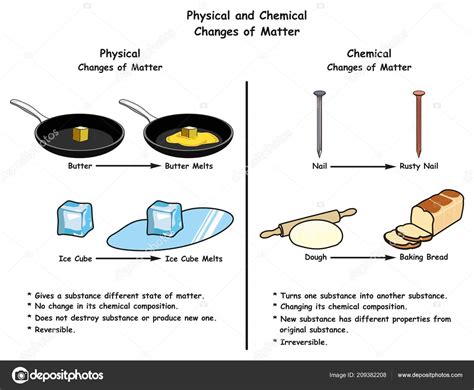
Table of Contents
Is Melting Butter a Physical or Chemical Change? A Deep Dive
The seemingly simple act of melting butter sparks a fascinating question in the world of chemistry: is it a physical or chemical change? At first glance, it might seem straightforward, but a closer examination reveals a more nuanced answer, one that delves into the intricate structure of butter and the processes involved in its transformation from solid to liquid. This article will explore the science behind melting butter, differentiating between physical and chemical changes, and ultimately answering the central question.
Understanding Physical and Chemical Changes
Before we delve into the specifics of melting butter, it's crucial to define the key terms: physical and chemical change.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same; only its physical properties (like shape, size, or state of matter) are modified. Examples include:
- Melting ice: Ice (solid water) melts into liquid water, but the chemical composition (H₂O) remains unchanged.
- Boiling water: Liquid water turns into water vapor (steam), but it's still H₂O.
- Dissolving sugar in water: The sugar disappears into the water, but it's still sugar; it's just dispersed.
These changes are often reversible. You can freeze liquid water back into ice, condense steam back into liquid water, and even recover the sugar through evaporation.
Chemical Changes: A Transformation of Substance
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances with different chemical properties. The original substance is transformed into something entirely different. Examples include:
- Burning wood: Wood reacts with oxygen in the air to produce ashes, smoke, and gases. The original wood is gone, replaced by entirely different compounds.
- Rusting iron: Iron reacts with oxygen and water to form iron oxide (rust), a new compound with different properties than iron.
- Baking a cake: The ingredients undergo chemical reactions, creating new compounds that give the cake its texture and flavor. You can't simply unbake a cake to get the original ingredients back.
These changes are generally irreversible or very difficult to reverse.
The Composition of Butter: A Complex Mixture
To understand whether melting butter is a physical or chemical change, we need to examine its composition. Butter isn't a single compound; it's a complex mixture of:
- Fats (Triglycerides): These constitute the majority of butter, typically around 80%. They are composed of glycerol and three fatty acids. The type of fatty acids present (saturated, unsaturated, etc.) influences the melting point and other properties of the butter.
- Water: Butter contains a significant amount of water, usually around 15-17%.
- Milk solids: These include proteins, lactose (milk sugar), and minerals. They contribute to the flavor and texture of butter.
The Melting Process: A Physical Transformation
When you melt butter, you're primarily breaking down the intermolecular forces holding the fat molecules together in a solid state. These forces are weak attractions between molecules, not strong chemical bonds within the molecules themselves. As heat is applied, the kinetic energy of the fat molecules increases, overcoming these weak forces and allowing them to move more freely, transitioning from a solid to a liquid state.
The water present in the butter also undergoes a physical change—it heats up and may even evaporate if the temperature is high enough. The milk solids remain largely unchanged; they may disperse more evenly in the melted butter, but their chemical structure stays intact.
Crucially, no new chemical compounds are formed during the melting process. The triglycerides, water, and milk solids are still present in the melted butter; they've simply changed their physical state. This is a key indicator of a physical change.
Addressing Potential Arguments Against a Purely Physical Change
Some might argue that the slight changes in flavor and aroma that can occur during butter melting represent a chemical change. However, these changes are typically due to:
- Volatilization of aromatic compounds: Some volatile aroma compounds might evaporate at higher temperatures, leading to a slight change in the butter's overall scent. This is a physical process (phase transition) rather than a chemical reaction.
- Maillard reaction (at high temperatures): At very high temperatures, the Maillard reaction, a complex series of chemical reactions between amino acids and reducing sugars, can occur, leading to browning and changes in flavor. However, this is only significant at temperatures far exceeding typical melting points. Normal butter melting avoids this.
- Hydrolysis (at high temperatures and prolonged heating): Prolonged heating at high temperatures can lead to hydrolysis of triglycerides, breaking them down into glycerol and fatty acids. However, this is not a characteristic of simple butter melting but rather a degradation process.
Conclusion: Melting Butter is Primarily a Physical Change
In conclusion, melting butter is primarily a physical change. The heat energy overcomes the weak intermolecular forces holding the fat molecules together, causing a transition from solid to liquid. While minor changes in aroma and flavor might occur due to volatilization, these are not indicative of a significant chemical transformation. The chemical composition of the butter remains largely unchanged. Only under extreme conditions of high temperature and prolonged heating do significant chemical changes start to occur. Therefore, for all practical purposes, the melting of butter is a classic example of a physical change.
Further Exploration: Exploring Related Concepts
Understanding the melting of butter can open doors to exploring related concepts:
- Melting points: Different fats have different melting points due to variations in their fatty acid composition. This impacts the properties of different types of butter and other fats.
- Phase transitions: Melting is just one type of phase transition; others include boiling, freezing, and sublimation.
- Intermolecular forces: Understanding the various types of intermolecular forces (Van der Waals forces, hydrogen bonding, etc.) helps explain the behavior of different substances during phase transitions.
- Food chemistry: The study of food chemistry provides deeper insights into the changes that occur during food preparation, including melting, cooking, and other processes.
By exploring these related concepts, we gain a more profound appreciation for the intricate science behind seemingly simple everyday occurrences. The melting of butter, a process we often take for granted, serves as an excellent introduction to the fascinating world of physical and chemical changes.
Latest Posts
Latest Posts
-
Wax Melting Physical Or Chemical Change
Mar 17, 2025
-
How Many Lines Of Symmetry Does This Equilateral Triangle Have
Mar 17, 2025
-
Which Of The Following Is The Poorest Conductor Of Electricity
Mar 17, 2025
-
What Is The Name Of A 7 Sided Polygon
Mar 17, 2025
-
What Is Difference Between Evaporation And Vaporization
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Melting Butter A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
