Wax Melting Physical Or Chemical Change
Juapaving
Mar 17, 2025 · 6 min read
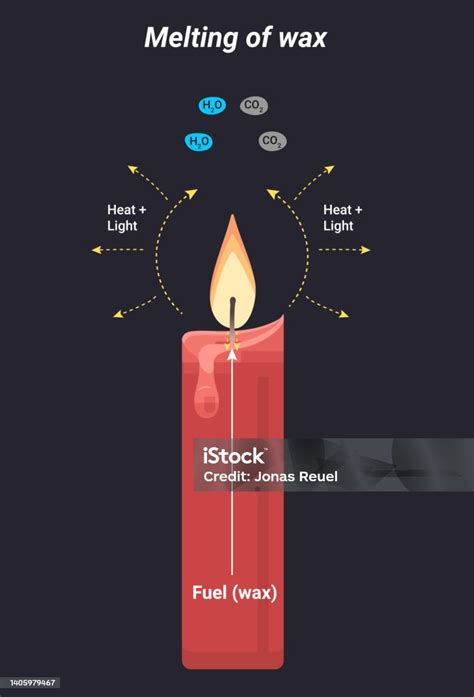
Table of Contents
Wax Melting: A Deep Dive into Physical Changes
Is melting wax a physical or chemical change? This seemingly simple question opens a door to a fascinating exploration of matter, its properties, and the processes that transform it. The answer, in short, is physical. But understanding why requires a closer look at the nature of physical and chemical changes, the properties of wax, and the molecular interactions involved in the melting process.
Understanding Physical and Chemical Changes
Before delving into the specifics of wax, let's establish a clear understanding of the fundamental difference between physical and chemical changes. This distinction lies in whether the fundamental chemical composition of the substance alters.
Physical Changes: A Change in Form, Not Substance
A physical change alters the form or appearance of a substance but doesn't change its chemical identity. Think about it like this: you can change the shape, size, or state (solid, liquid, gas) of a substance, but the molecules themselves remain the same. Examples include:
- Melting ice: Ice (solid water) melts into liquid water, but the water molecules are unchanged.
- Boiling water: Liquid water turns into water vapor (gas), again without altering the water molecule.
- Dissolving sugar in water: The sugar molecules disperse in the water, but they remain sugar molecules.
- Crushing a can: The shape of the can changes, but the aluminum remains aluminum.
Chemical Changes: A Change in Composition
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances with different chemical properties. The original substance is fundamentally transformed. Examples include:
- Burning wood: Wood reacts with oxygen to produce ash, smoke, and gases – entirely new substances.
- Rusting iron: Iron reacts with oxygen and water to form iron oxide (rust), a different compound.
- Baking a cake: The ingredients undergo a series of chemical reactions to create a new substance with different properties.
- Digestion: The complex molecules in food are broken down into simpler molecules through chemical reactions.
The Nature of Wax
Wax, in its various forms (paraffin wax, beeswax, soy wax, etc.), is a complex mixture of hydrocarbons. These hydrocarbons are long chains of carbon atoms bonded to hydrogen atoms. The specific composition and length of these chains vary depending on the type of wax. This variation in composition is why different waxes have different melting points, textures, and other properties. For example, beeswax is a natural wax with a complex mixture of esters, while paraffin wax is a refined petroleum product with a more uniform composition of long-chain hydrocarbons.
The Molecular Structure of Wax and its Implications
The hydrocarbon chains in wax are held together by relatively weak intermolecular forces, primarily van der Waals forces. These forces are much weaker than the covalent bonds that hold the atoms within each hydrocarbon molecule together. This is crucial to understanding the melting process.
Melting Wax: A Detailed Explanation
When wax is heated, the increased thermal energy causes the molecules to vibrate more vigorously. This increased vibration overcomes the weak intermolecular forces holding the molecules together in the solid state. As the temperature reaches the melting point, the wax transitions from a solid to a liquid.
The Role of Intermolecular Forces
The relatively weak intermolecular forces in wax are easily overcome by the increased kinetic energy of the molecules at higher temperatures. This is why wax has a relatively low melting point compared to substances with stronger intermolecular forces. The transition from solid to liquid is a gradual process, not an abrupt change. As more and more molecules gain enough energy to overcome the intermolecular forces, the wax progressively softens and eventually flows like a liquid.
No New Substances are Formed
Critically, throughout the melting process, the individual hydrocarbon molecules remain intact. There is no breaking or formation of covalent bonds within the molecules themselves. The only change is in the arrangement and interactions between the molecules. They transition from a relatively ordered, solid state to a more disordered, liquid state. This lack of change in chemical composition is the hallmark of a physical change.
The Cooling and Solidification Process: A Reversible Physical Change
The melting of wax is a reversible process. When the liquid wax cools, the molecules lose kinetic energy, and the intermolecular forces begin to reassert themselves. The molecules gradually become more organized, eventually forming the solid crystalline structure characteristic of wax. This transition from liquid to solid is also a physical change, further reinforcing the nature of melting as a physical process.
Different Types of Wax and Their Melting Points
The specific melting point of wax depends on its composition. Different types of waxes have different hydrocarbon chain lengths and structures, leading to variations in their melting points. Generally:
- Paraffin wax: Commonly has a melting point range of 46-68°C (115-154°F).
- Beeswax: Typically melts between 62-72°C (144-162°F).
- Soy wax: Usually melts between 45-54°C (113-129°F).
These variations in melting points are due to the differences in intermolecular forces between the different wax types. Longer hydrocarbon chains generally lead to stronger van der Waals forces and higher melting points.
Practical Applications and Considerations
The melting and solidification of wax have numerous practical applications, including:
- Candle making: Wax is melted and poured into molds to create candles.
- Cosmetics and skincare: Waxes are used as emulsifiers and thickeners in various cosmetic products.
- Food industry: Certain waxes are used as coatings for fruits and vegetables.
- Industrial applications: Waxes are used as lubricants, coatings, and in various other industrial processes.
Understanding the physical nature of wax melting is crucial for optimizing these applications. Controlling the temperature and cooling rate allows for precise control over the final properties of the wax product.
Misconceptions about Wax Melting
There is often a misunderstanding about the burning of candles and whether that's a chemical or physical change. While melting the wax itself is a physical change, the burning of the candle wick is a chemical change. The burning process involves a chemical reaction between the wax and oxygen in the air, producing carbon dioxide, water vapor, and other byproducts. This combustion process fundamentally alters the chemical composition of the wax.
Conclusion: A Physical Transformation
In conclusion, melting wax is unequivocally a physical change. The process involves only a change in the state of matter, from solid to liquid, without altering the chemical composition of the wax itself. The relatively weak intermolecular forces within the wax are overcome by the increased kinetic energy of the molecules at elevated temperatures. This understanding is crucial for various applications involving wax, from candle making to industrial processes. While the combustion of wax during candle burning is a chemical change, the melting of the wax prior to burning remains firmly within the realm of physical transformations. The reversible nature of the melting and solidification process further underscores its physical character. This detailed exploration clarifies the fundamental distinction between physical and chemical changes and highlights the importance of understanding molecular interactions in explaining macroscopic phenomena.
Latest Posts
Latest Posts
-
Multiples Of 5 Up To 100
Mar 17, 2025
-
What Is The Difference Between A Solute And A Solvent
Mar 17, 2025
-
How To Prove A Square Is A Square
Mar 17, 2025
-
Is 34 An Even Or Odd Number
Mar 17, 2025
-
Which Of The Following Is An Example Of A Decomposer
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Wax Melting Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
