How Many Valence Shell Electrons Does The Element Carbon Have
Juapaving
Mar 14, 2025 · 5 min read
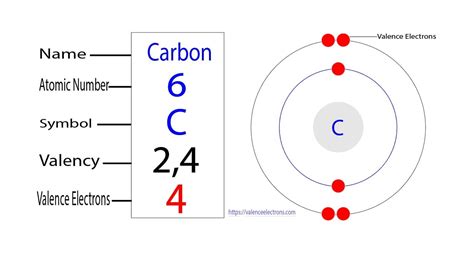
Table of Contents
How Many Valence Shell Electrons Does the Element Carbon Have? Understanding Carbon's Bonding Prowess
Carbon, the cornerstone of organic chemistry and the building block of life as we know it, possesses a unique electronic structure that dictates its remarkable bonding capabilities. Understanding the number of valence electrons in carbon is fundamental to grasping its reactivity and the vast diversity of molecules it forms. This article delves deep into the electronic configuration of carbon, explaining its valence electrons and their profound influence on its chemical behavior.
Carbon's Atomic Structure: Unveiling the Secrets
To understand carbon's valence electrons, we must first examine its atomic structure. Carbon (C) has an atomic number of 6, meaning it possesses six protons and six electrons in its neutral state. These electrons are arranged in specific energy levels or shells surrounding the nucleus.
Electron Shell Configuration: A Closer Look
- Shell 1 (K-shell): This innermost shell can accommodate a maximum of two electrons. In carbon, both electrons occupy this shell.
- Shell 2 (L-shell): This outer shell holds the remaining four electrons. These four electrons are crucial because they determine carbon's chemical behavior.
Defining Valence Electrons: The Chemical Actors
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound and readily participate in chemical bonding. They are the key players in determining an element's reactivity and the types of chemical bonds it can form.
Carbon's Valence Electrons: The Four Key Players
Given carbon's electron configuration (1s²2s²2p²), it has four valence electrons located in its outermost (L) shell. These four electrons are responsible for carbon's exceptional ability to form strong covalent bonds with a wide range of elements.
Carbon's Bonding Capabilities: A Testament to its Valence Electrons
The presence of four valence electrons allows carbon to achieve a stable octet configuration (eight electrons in its outermost shell) through several bonding mechanisms. This drive for stability is the driving force behind carbon's extensive chemical diversity.
Covalent Bonding: Sharing is Caring
Carbon most commonly forms covalent bonds. In a covalent bond, atoms share one or more pairs of valence electrons to achieve a stable electron configuration. Because carbon has four valence electrons, it can form up to four covalent bonds. This ability underpins the vast complexity of organic molecules.
Examples of Covalent Bonding in Carbon:
- Methane (CH₄): Carbon forms four single covalent bonds with four hydrogen atoms, each sharing one electron pair.
- Ethane (C₂H₆): Two carbon atoms form a single covalent bond with each other, sharing one electron pair. Each carbon atom then forms three additional single covalent bonds with hydrogen atoms.
- Ethene (C₂H₄): Two carbon atoms form a double covalent bond by sharing two electron pairs, in addition to single bonds with hydrogen atoms.
- Ethyne (C₂H₂): Two carbon atoms form a triple covalent bond by sharing three electron pairs, along with single bonds to hydrogen atoms.
Carbon's Versatility: Chain Formation and Ring Structures
The ability of carbon to form four covalent bonds leads to the formation of long chains and ring structures. These chains and rings are the backbone of many organic molecules, creating the incredible diversity of compounds found in living organisms and synthetic materials.
Examples of Carbon Chain and Ring Structures:
- Linear chains: Found in alkanes like butane and octane.
- Branched chains: Seen in isobutane and other isomers.
- Ring structures: Present in cyclohexane, benzene, and many other cyclic compounds.
The Significance of Carbon's Valence Electrons in Organic Chemistry
Carbon's four valence electrons are the fundamental reason behind the vast field of organic chemistry. The ability to form stable and diverse covalent bonds allows carbon to create an almost infinite number of different molecules with varying structures, properties, and functions. This is why carbon is the central element in the chemistry of life.
The Basis of Life's Building Blocks:
- Carbohydrates: Composed of carbon, hydrogen, and oxygen, carbohydrates serve as a primary energy source for living organisms. The carbon atoms form the backbone of these molecules.
- Lipids: Fats, oils, and waxes are all lipids, crucial for energy storage, cell membrane structure, and hormone production. The carbon backbone again plays a pivotal role.
- Proteins: Proteins are essential for numerous biological functions, including enzymatic catalysis, structural support, and transportation. The amino acids that make up proteins contain carbon atoms as a fundamental part of their structure.
- Nucleic Acids (DNA and RNA): These molecules carry the genetic information essential for life. The carbon atoms form the sugar-phosphate backbone of DNA and RNA, with nitrogenous bases attached to the carbons.
Beyond Organic Chemistry: Carbon's Wider Impact
The importance of carbon's four valence electrons isn't limited to organic chemistry. Carbon's bonding versatility extends to inorganic compounds as well. It forms various compounds with other elements, exhibiting diverse properties.
Inorganic Carbon Compounds:
- Carbon dioxide (CO₂): A crucial greenhouse gas, carbon dioxide plays a vital role in the Earth's climate system.
- Carbon monoxide (CO): A toxic gas, carbon monoxide is a byproduct of incomplete combustion.
- Carbonates (CO₃²⁻): Carbonates are found in various minerals, such as limestone and marble.
Conclusion: The Remarkable Chemistry of Carbon
The number of valence electrons an atom possesses dictates its chemical behavior. Carbon, with its four valence electrons, stands as a testament to this principle. Its remarkable ability to form strong and diverse covalent bonds is the foundation of organic chemistry and underpins the complexity and diversity of life itself. From the simplest hydrocarbons to the intricate macromolecules of living organisms, carbon's four valence electrons are the driving force behind a vast and fascinating realm of chemical possibilities. Understanding this fundamental aspect of carbon's atomic structure provides a crucial insight into the world around us. The study of carbon's valence electrons remains a cornerstone of chemical research, constantly unlocking new insights and applications in various fields, ranging from materials science to medicine. The multifaceted nature of carbon's chemistry continues to be a source of inspiration and innovation for scientists worldwide.
Latest Posts
Latest Posts
-
What Does Sound Travel Slowest Through
Mar 14, 2025
-
What State Of Matter Takes The Shape Of Its Container
Mar 14, 2025
-
What Is All The Factors Of 56
Mar 14, 2025
-
What Gas Is Released During Photosynthesis
Mar 14, 2025
-
Round The Number To The Nearest Thousandth
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Shell Electrons Does The Element Carbon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
