Is Glucose Oxidized Or Reduced In Cellular Respiration
Juapaving
Mar 29, 2025 · 6 min read
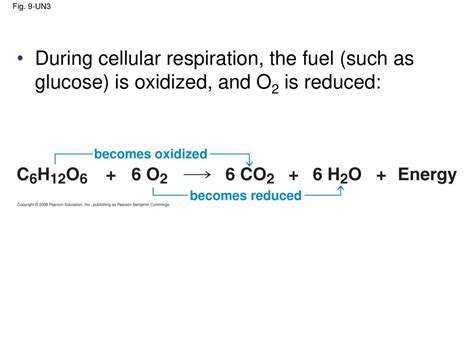
Table of Contents
- Is Glucose Oxidized Or Reduced In Cellular Respiration
- Table of Contents
- Is Glucose Oxidized or Reduced in Cellular Respiration? Understanding Redox Reactions in Metabolism
- Understanding Oxidation and Reduction
- Oxidation: The Loss of Electrons
- Reduction: The Gain of Electrons
- Cellular Respiration: A Cascade of Redox Reactions
- Glycolysis: The Initial Oxidation Steps
- Key Oxidation Events in Glycolysis:
- Pyruvate Oxidation: Linking Glycolysis to the Citric Acid Cycle
- Key Oxidation Events in Pyruvate Oxidation:
- The Citric Acid Cycle: Central Hub of Redox Reactions
- Key Oxidation Events in the Citric Acid Cycle:
- Oxidative Phosphorylation: Harvesting Energy from Electrons
- Key Oxidation Events in Oxidative Phosphorylation:
- Conclusion: Glucose's Oxidative Journey
- Latest Posts
- Latest Posts
- Related Post
Is Glucose Oxidized or Reduced in Cellular Respiration? Understanding Redox Reactions in Metabolism
Cellular respiration, the process by which cells break down glucose to generate energy in the form of ATP, is a cornerstone of life. A crucial aspect of this complex process involves redox reactions, where electrons are transferred between molecules. A key question that arises is whether glucose is oxidized or reduced during cellular respiration. The answer, as we'll explore in detail, is that glucose is oxidized. This article delves deep into the intricacies of redox reactions, tracing the journey of electrons from glucose throughout the various stages of cellular respiration, and clarifies the critical role of oxidation in energy production.
Understanding Oxidation and Reduction
Before diving into the specifics of glucose's fate in cellular respiration, it's vital to establish a solid understanding of oxidation and reduction (redox) reactions. These reactions always occur in pairs: one molecule is oxidized (loses electrons), while another is reduced (gains electrons). Remembering the mnemonic "OIL RIG" – Oxidation Is Loss, Reduction Is Gain – can be helpful.
Oxidation: The Loss of Electrons
Oxidation involves the loss of electrons from a molecule. This can manifest in several ways:
- Direct electron transfer: A molecule directly donates electrons to another molecule.
- Hydrogen atom loss: The loss of a hydrogen atom (H) is effectively the loss of an electron (since H atom consists of one proton and one electron) and a proton (H⁺).
- Oxygen atom gain: While not strictly an electron loss, the addition of an oxygen atom often involves a shift in electron distribution, resulting in a more oxidized state. This is because oxygen is highly electronegative, attracting electrons towards itself.
Reduction: The Gain of Electrons
Reduction is the complementary process, where a molecule gains electrons. This can also occur through:
- Direct electron transfer: A molecule accepts electrons from another molecule.
- Hydrogen atom gain: The gain of a hydrogen atom effectively means a gain of an electron and a proton.
- Oxygen atom loss: Similar to oxidation, the removal of an oxygen atom frequently involves a change in electron distribution, resulting in a more reduced state.
Cellular Respiration: A Cascade of Redox Reactions
Cellular respiration is a multi-step process that can be broadly divided into four main stages: glycolysis, pyruvate oxidation, the citric acid cycle (Krebs cycle), and oxidative phosphorylation (electron transport chain and chemiosmosis). Throughout these stages, glucose undergoes a series of redox reactions, ultimately resulting in its complete oxidation.
Glycolysis: The Initial Oxidation Steps
Glycolysis, occurring in the cytoplasm, is the first step in glucose metabolism. Although it doesn't require oxygen (anaerobic), it still involves oxidation-reduction reactions. The initial glucose molecule (a highly reduced molecule with many C-H bonds) undergoes a series of enzymatic reactions.
Key Oxidation Events in Glycolysis:
- Glyceraldehyde-3-phosphate (G3P) oxidation: This is a pivotal step. G3P is oxidized by the enzyme glyceraldehyde-3-phosphate dehydrogenase. In this reaction, two electrons and a proton are transferred to NAD⁺, reducing it to NADH. Simultaneously, G3P is oxidized, forming a higher-energy molecule that will eventually yield ATP. This is a crucial redox reaction, capturing electrons from glucose in a usable form (NADH).
- Formation of pyruvate: The final product of glycolysis, pyruvate, is a more oxidized molecule than glucose. It has fewer C-H bonds and a higher number of C=O bonds, reflecting the loss of electrons.
Pyruvate Oxidation: Linking Glycolysis to the Citric Acid Cycle
Pyruvate, generated in glycolysis, is transported into the mitochondria, where it undergoes further oxidation. The process of pyruvate oxidation converts pyruvate into acetyl-CoA, a crucial molecule for the citric acid cycle.
Key Oxidation Events in Pyruvate Oxidation:
- Decarboxylation: A carbon atom is removed from pyruvate as carbon dioxide (CO₂), a fully oxidized form of carbon.
- Acetyl-CoA formation: The remaining two-carbon acetyl group is attached to coenzyme A (CoA), forming acetyl-CoA. This process also involves a redox reaction where NAD⁺ is reduced to NADH.
The Citric Acid Cycle: Central Hub of Redox Reactions
The citric acid cycle (also known as the Krebs cycle or tricarboxylic acid cycle) takes place within the mitochondrial matrix. This cyclical pathway further oxidizes the acetyl group from acetyl-CoA, generating high-energy electron carriers and releasing CO₂.
Key Oxidation Events in the Citric Acid Cycle:
- Acetyl-CoA oxidation: The acetyl group (two carbons) is completely oxidized, releasing two molecules of CO₂. Electrons are transferred to NAD⁺ (forming NADH) and FAD (forming FADH₂), both electron carriers.
- Multiple redox reactions: Each cycle involves multiple oxidation and reduction steps, with the progressive oxidation of carbon atoms and the reduction of electron carriers. These steps involve enzymes such as isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and succinate dehydrogenase, each catalyzing a specific redox reaction.
Oxidative Phosphorylation: Harvesting Energy from Electrons
Oxidative phosphorylation is the final stage of cellular respiration, responsible for generating the majority of ATP. This stage consists of two coupled processes: the electron transport chain and chemiosmosis. The electron carriers NADH and FADH₂, generated in the previous stages, are crucial here.
Key Oxidation Events in Oxidative Phosphorylation:
- Electron transport chain: Electrons from NADH and FADH₂ are passed down a series of protein complexes embedded in the inner mitochondrial membrane. As electrons move along the chain, they lose energy, which is used to pump protons (H⁺) across the membrane, creating a proton gradient. The final electron acceptor is oxygen (O₂), which is reduced to water (H₂O). The oxidation of NADH and FADH₂ fuels the entire process.
- Chemiosmosis: The proton gradient created by the electron transport chain drives ATP synthesis through chemiosmosis. Protons flow back across the membrane through ATP synthase, an enzyme that uses the energy of the proton flow to phosphorylate ADP to ATP.
Conclusion: Glucose's Oxidative Journey
The detailed analysis of the four stages of cellular respiration reveals a clear narrative: glucose is oxidized. Starting as a highly reduced molecule rich in C-H bonds, it undergoes a stepwise oxidation, releasing electrons that are captured by electron carriers (NADH and FADH₂). These electrons are then used in the electron transport chain to generate a proton gradient, driving ATP synthesis. The ultimate products of the complete oxidation of glucose are CO₂ (fully oxidized carbon) and H₂O (reduced oxygen). The energy released during this oxidative process is harnessed to produce ATP, the cell's primary energy currency. Understanding the oxidation of glucose and the role of redox reactions in cellular respiration is essential for comprehending the fundamental processes of life and energy metabolism. The intricate coordination of enzymes, electron carriers, and membrane-bound complexes ensures a highly efficient energy harvesting system, underpinning the survival and function of all aerobic organisms. Future research continues to unravel the fine details and regulatory mechanisms within this incredibly vital metabolic pathway.
Latest Posts
Latest Posts
-
Air Moves From High To Low Pressure
Apr 02, 2025
-
The Smallest Particle Of An Element Is A N
Apr 02, 2025
-
How Many Neutrons Does A Hydrogen Atom Have
Apr 02, 2025
-
What Is Nickel Used For In Everyday Life
Apr 02, 2025
-
A Tetrad Is Made Up Of
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Glucose Oxidized Or Reduced In Cellular Respiration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
