Is Frying An Egg A Chemical Change
Juapaving
Mar 20, 2025 · 5 min read
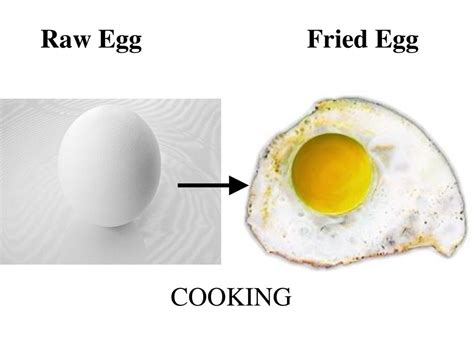
Table of Contents
Is Frying an Egg a Chemical Change? A Deep Dive into Culinary Chemistry
Frying an egg is a commonplace activity, a breakfast staple for millions. But have you ever stopped to consider the deeper scientific processes at play? Is this seemingly simple act merely a physical transformation, or does it represent a fundamental chemical change? The answer, as we'll explore, is far more nuanced than a simple yes or no. This article will delve into the fascinating chemistry behind frying an egg, examining the structural changes, irreversible reactions, and overall evidence supporting its classification as a chemical change.
Understanding Chemical vs. Physical Changes
Before we embark on our investigation of the fried egg, let's establish a clear understanding of the difference between chemical and physical changes.
Physical changes alter the form or appearance of a substance without changing its chemical composition. Think of melting ice – it changes from a solid to a liquid, but it remains H₂O. Other examples include dissolving sugar in water, cutting paper, or boiling water. These changes are often reversible.
Chemical changes, on the other hand, involve the creation of new substances with different chemical properties. These changes are often irreversible and are accompanied by observable signs such as a change in color, odor, temperature, or the formation of a gas or precipitate. Burning wood, rusting iron, and baking a cake are all examples of chemical changes.
The Science of a Frying Egg: A Step-by-Step Analysis
Now, let's break down the process of frying an egg to analyze whether it fits the criteria for a chemical change.
1. The Egg's Initial Composition: A Complex Mixture
A raw egg is a complex mixture of various components:
- The White (Albumen): Primarily composed of water (around 90%), protein (primarily ovalbumin, ovotransferrin, ovomucoid, and lysozyme), and small amounts of glucose and minerals.
- The Yolk: A concentrated emulsion of water, lipids (fats), proteins (lipoproteins and phospholipids), and various vitamins and minerals. The yolk’s color comes from carotenoid pigments.
2. The Heat is On: Denaturation Begins
When we apply heat to the egg, the magic begins. The key player here is protein denaturation. Proteins are complex molecules with intricate three-dimensional structures held together by weak bonds like hydrogen bonds and disulfide bridges. Heat disrupts these weak bonds, causing the protein molecules to unfold and lose their original shape. This process is called denaturation.
3. Irreversible Changes: Coagulation and Color Alterations
As denaturation progresses, the unfolded proteins begin to interact with each other, forming new bonds and creating a more solid, interconnected network. This process is known as coagulation. The liquid egg white and yolk transform into a solid, opaque mass. This change is irreversible. You cannot simply cool the cooked egg to return it to its original liquid state.
The application of heat also causes the Maillard reaction, a complex series of chemical reactions between amino acids and reducing sugars. This reaction is responsible for the characteristic browning and development of flavor and aroma in many cooked foods, including the fried egg. The Maillard reaction is a further indication of a chemical transformation as new molecules are formed, resulting in a change in color and flavor profile.
4. Lipid Changes: From Liquid to Solid
The yolk's lipids also undergo a change. Initially, these fats are liquid at room temperature. However, during frying, the heat causes them to solidify to some extent, contributing to the overall change in texture and consistency of the cooked egg. This change, while partially reversible upon cooling (the fat will remain fat), represents an alteration in its physical state due to temperature, further adding to the overall evidence of chemical change.
5. Evidence of Chemical Change: Irreversibility and New Properties
Several observations clearly demonstrate that frying an egg is a chemical change:
- Irreversibility: As mentioned before, the cooked egg cannot be returned to its original raw state. This is a hallmark of chemical change.
- Color Change: The raw egg's translucent white and yellow yolk transform into opaque white and a darker yellow, respectively, indicating a change in chemical structure and composition.
- Texture Change: The liquid egg transforms into a solid, rubbery texture, indicative of new molecular bonds forming.
- Odor Change: The cooked egg has a distinct aroma different from the raw egg, a direct result of the Maillard reaction producing volatile aromatic compounds.
- Formation of New Compounds: The Maillard reaction, itself, creates numerous new flavor and aroma compounds, solidifying the concept of chemical transformation.
Addressing Potential Counterarguments
Some might argue that the changes observed are merely physical, pointing to the fact that the basic chemical components – proteins, fats, and water – remain the same. However, the arrangement and bonding of these components undergo a drastic and irreversible alteration. This rearrangement, the formation of new compounds (Maillard reaction products), and the creation of a significantly altered structure clearly signifies a chemical change. The egg's properties have fundamentally changed, even if the individual elements remain the same. This is the core difference between a physical and chemical change.
Conclusion: The Fried Egg as a Chemical Marvel
In conclusion, overwhelming evidence supports the assertion that frying an egg is a chemical change. The denaturation of proteins, the Maillard reaction, the changes in lipid state, the irreversible nature of the transformation, and the creation of new compounds all point to this fact. While the fundamental elements of the egg remain, their organization and bonding have irrevocably altered, producing a substance with entirely different properties. The next time you enjoy a fried egg, remember the fascinating chemical reactions taking place before your eyes – a small, delicious example of the incredible complexity of culinary chemistry.
Further Exploration: Expanding Your Culinary Chemistry Knowledge
This exploration only scratches the surface of the fascinating chemistry behind cooking. For a deeper dive, consider researching the following topics:
- The Maillard reaction: Explore the specific reactions involved and the factors influencing its outcome.
- Protein structure and denaturation: Understand the different types of protein bonds and how heat affects them.
- Lipid oxidation: Investigate how fats react to heat and the role of antioxidants.
- Enzyme activity in cooking: Explore how heat affects enzymes present in food, impacting flavor and texture.
By understanding the science behind our everyday culinary practices, we can appreciate not only the delicious results but also the intricate chemical processes that make them possible. Frying an egg is more than just breakfast; it's a miniature chemical experiment, demonstrating the transformative power of heat and the dynamic interplay of molecules.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 8 And 9
Mar 20, 2025
-
A Measure Of The Quantity Of Matter Is
Mar 20, 2025
-
Is Rusting Of Iron A Chemical Change
Mar 20, 2025
-
Which Of The Following Is An Extensive Property Of Matter
Mar 20, 2025
-
Sum Of Exterior Angles Of A Hexagon
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Frying An Egg A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
