Is Enzyme A Carbohydrate Protein Lipid Or Nucleic Acid
Juapaving
Mar 30, 2025 · 6 min read
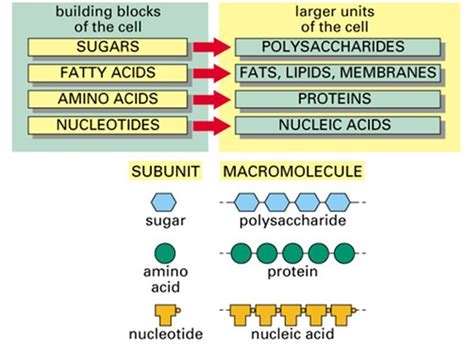
Table of Contents
Is an Enzyme a Carbohydrate, Protein, Lipid, or Nucleic Acid?
Enzymes are fundamental to life, driving countless biochemical reactions within organisms. Understanding their composition is crucial to comprehending their function and the intricate machinery of living systems. This comprehensive article will delve into the chemical nature of enzymes, definitively answering the question: Is an enzyme a carbohydrate, protein, lipid, or nucleic acid? We'll also explore the properties that make enzymes so uniquely effective biological catalysts.
Enzymes: The Biological Catalysts
Before we pinpoint the specific class of biomolecules to which enzymes belong, let's establish a foundational understanding of their role. Enzymes are biological catalysts, meaning they accelerate the rate of chemical reactions without being consumed in the process. Their remarkable efficiency allows life's complex processes to occur at speeds compatible with survival. Without enzymes, many essential reactions would proceed far too slowly to sustain life.
Think of enzymes as highly specialized molecular machines. Each enzyme is designed to interact with a specific molecule, called a substrate. This interaction occurs at a specific region on the enzyme known as the active site. The active site possesses a unique three-dimensional structure that complements the shape and chemical properties of the substrate, enabling them to bind with remarkable specificity. This lock-and-key model, while simplified, provides a useful visual analogy for understanding enzyme-substrate interactions. A more nuanced model, the induced-fit model, considers the dynamic nature of enzyme-substrate binding, where the enzyme's active site undergoes conformational changes upon substrate binding, optimizing the interaction for catalysis.
The Composition of Enzymes: Unmasking the Truth
Now, let's address the core question: What type of biomolecule is an enzyme? The answer is definitively protein. While a tiny minority of catalytic RNA molecules, known as ribozymes, exhibit enzymatic activity, the vast majority of enzymes are proteins.
This protein nature dictates their properties and functionality. Proteins are constructed from chains of amino acids, linked together through peptide bonds. The sequence of these amino acids determines the enzyme's unique three-dimensional structure, which is crucial for its catalytic activity. The precise folding pattern creates the active site, ensuring the enzyme binds to its specific substrate(s) and facilitates the desired chemical transformation.
Let's examine why enzymes are not carbohydrates, lipids, or nucleic acids:
Enzymes are NOT Carbohydrates
Carbohydrates are primarily composed of carbon, hydrogen, and oxygen atoms, typically in a ratio of 1:2:1. They serve as primary energy sources and structural components in cells. While some carbohydrates can participate in enzymatic reactions as substrates or modifiers, they do not possess the inherent catalytic properties that define enzymes. Their structural rigidity and lack of diverse functional groups prevent them from adopting the complex three-dimensional structures necessary for enzyme function.
Enzymes are NOT Lipids
Lipids are a diverse group of hydrophobic molecules, including fats, oils, waxes, and steroids. Their primary functions involve energy storage, membrane structure, and hormone signaling. Lipids generally lack the functional groups necessary for the precise interactions required in enzyme catalysis. While some lipid-modifying enzymes exist, the enzymes themselves are not composed of lipids. The hydrophobic nature of many lipids also clashes with the hydrophilic environment of most enzyme-catalyzed reactions within cells.
Enzymes are NOT Nucleic Acids
Nucleic acids, DNA and RNA, carry the genetic information of cells. While ribozymes, catalytic RNA molecules, represent a notable exception to the protein-only rule for enzymes, they still represent a small fraction of the total enzymatic activity within a cell. The vast majority of enzymes are proteins, exhibiting far greater diversity and complexity in their catalytic capabilities than ribozymes. DNA, in particular, lacks the capacity for the intricate folding and dynamic interactions needed for enzyme function. Its primary role is the storage and transmission of genetic information, not catalysis.
The Importance of Protein Structure in Enzyme Function
The protein nature of most enzymes is inextricably linked to their function. The precise three-dimensional structure of a protein enzyme, arising from its amino acid sequence, is paramount for its catalytic activity. This structure is stabilized by a variety of interactions, including:
- Peptide bonds: The covalent bonds linking amino acids together form the backbone of the protein.
- Hydrogen bonds: These relatively weak bonds contribute significantly to the overall folding pattern of the protein.
- Disulfide bridges: Covalent bonds between cysteine residues create strong cross-links, stabilizing the protein's structure.
- Hydrophobic interactions: Nonpolar amino acid side chains tend to cluster together in the protein's core, minimizing their contact with the surrounding aqueous environment.
- Ionic interactions: Attractive forces between oppositely charged amino acid side chains contribute to the protein's three-dimensional stability.
Any disruption to this carefully orchestrated structure, such as through changes in temperature, pH, or the presence of denaturants, can significantly impact or completely abolish enzyme activity. This highlights the critical link between protein structure and enzyme function. The specificity of the active site, essential for recognizing and binding the substrate, is entirely dependent on this precise three-dimensional arrangement of amino acids.
Factors Affecting Enzyme Activity
Several factors influence the rate of enzyme-catalyzed reactions:
Substrate Concentration:
At low substrate concentrations, increasing the substrate concentration increases the reaction rate proportionally. However, at high substrate concentrations, the reaction rate plateaus, as all enzyme active sites become saturated with substrate.
Enzyme Concentration:
Increasing the concentration of enzymes increases the reaction rate, as more active sites are available to bind substrates.
Temperature:
Enzymes generally exhibit optimal activity within a specific temperature range. Increasing temperature initially increases reaction rate due to increased kinetic energy. However, excessively high temperatures can denature the enzyme, destroying its three-dimensional structure and abolishing activity.
pH:
Similar to temperature, enzymes have optimal pH ranges for activity. Extreme pH values can disrupt the electrostatic interactions within the enzyme, leading to denaturation and loss of function.
Inhibitors and Activators:
Inhibitors are molecules that reduce enzyme activity, either by binding to the active site (competitive inhibition) or to a different site on the enzyme (noncompetitive inhibition). Activators enhance enzyme activity, often by binding to regulatory sites and inducing conformational changes that increase substrate binding or catalytic efficiency.
Enzymes and Human Health
Enzymes are crucial for numerous physiological processes:
- Digestion: Digestive enzymes break down food into absorbable nutrients.
- Metabolism: Enzymes regulate metabolic pathways, controlling energy production and utilization.
- DNA replication and repair: Enzymes are essential for copying and repairing DNA.
- Immune function: Enzymes participate in immune responses, attacking pathogens and clearing cellular debris.
- Blood clotting: Enzymes are essential components of the blood clotting cascade.
Dysfunction of enzymes can lead to various diseases. Genetic mutations can cause the production of non-functional enzymes, resulting in metabolic disorders. Enzyme deficiencies can also be caused by environmental factors, such as exposure to toxins or nutrient deficiencies.
Conclusion: Enzymes are Primarily Proteins, the Essential Workhorses of Life
In conclusion, the answer is clear: enzymes are predominantly proteins. While some catalytic RNA molecules exist, the vast majority of enzymes are proteins, utilizing their intricate three-dimensional structures to catalyze the biochemical reactions that underpin life. Understanding their protein composition, structure-function relationship, and the factors influencing their activity is fundamental to comprehending the complexities of biological systems and developing effective strategies for treating enzyme-related diseases. The remarkable specificity and efficiency of enzymes make them indispensable for all forms of life, highlighting their significance as the essential workhorses of the biological world.
Latest Posts
Latest Posts
-
Examples Of The Eight Parts Of Speech
Apr 01, 2025
-
Is Anything That Occupies Space And Has Mass
Apr 01, 2025
-
Mario Has To Take Medication For 180 Days
Apr 01, 2025
-
What Is On A Physical Map
Apr 01, 2025
-
P Block Elements In Periodic Table
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Enzyme A Carbohydrate Protein Lipid Or Nucleic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
