What Is The Boiling Point And Freezing Point Of Water
Juapaving
Mar 26, 2025 · 6 min read
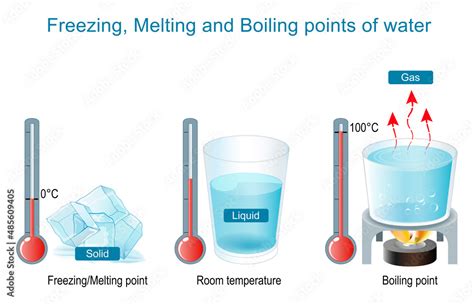
Table of Contents
What is the Boiling Point and Freezing Point of Water? A Deep Dive
Water, the elixir of life, is a substance so ubiquitous that we often take its properties for granted. Yet, understanding the fundamental characteristics of water, such as its boiling point and freezing point, is crucial in countless scientific disciplines and everyday life. This comprehensive guide delves deep into the physics and chemistry behind these critical temperature points, exploring their significance and the factors that can influence them.
Understanding Phase Transitions: From Solid to Liquid to Gas
Before we dive into the specifics of water's boiling and freezing points, let's establish a foundational understanding of phase transitions. Water, like all matter, can exist in three primary phases: solid (ice), liquid (water), and gas (water vapor or steam). These phases are dictated by the kinetic energy of the water molecules.
-
Solid (Ice): In the solid phase, water molecules are tightly bound together in a crystalline structure, characterized by strong intermolecular forces (hydrogen bonds). Their kinetic energy is relatively low, resulting in limited movement.
-
Liquid (Water): As heat is added, the kinetic energy of the water molecules increases, overcoming some of the intermolecular forces. The molecules become more mobile, allowing the water to flow and take the shape of its container.
-
Gas (Water Vapor/Steam): With sufficient heat, the kinetic energy overcomes the intermolecular forces entirely, allowing the water molecules to escape the liquid phase and exist as individual molecules in the gaseous phase. They move freely and randomly.
The transitions between these phases occur at specific temperatures under standard pressure conditions, which are the boiling and freezing points.
The Freezing Point of Water: 0°C (32°F)
The freezing point of water, the temperature at which liquid water transitions to solid ice, is precisely 0°C (32°F) at standard atmospheric pressure (1 atmosphere or 101.325 kPa). At this point, the kinetic energy of the water molecules decreases to a level where the intermolecular forces can effectively hold them in a fixed crystalline structure. This transition is an exothermic process, meaning it releases heat.
Factors Affecting the Freezing Point of Water:
While 0°C is the standard freezing point, several factors can influence the actual temperature at which water freezes:
-
Pressure: Increasing pressure slightly lowers the freezing point of water. This is a unique property of water, unlike most other substances.
-
Impurities: Dissolved substances, such as salts or sugars, lower the freezing point of water. This is the principle behind using salt to de-ice roads in winter. The salt dissolves in the liquid water, disrupting the formation of ice crystals and lowering the freezing temperature.
-
Supercooling: Under certain conditions, water can be cooled below 0°C without freezing. This phenomenon, known as supercooling, occurs when there are few nucleation sites (imperfections or surfaces) for ice crystals to form. A slight disturbance, such as a vibration or the addition of a small ice crystal, can trigger the rapid freezing of the supercooled water.
The Boiling Point of Water: 100°C (212°F)
The boiling point of water, the temperature at which liquid water transitions to gaseous steam, is 100°C (212°F) at standard atmospheric pressure. At this temperature, the kinetic energy of the water molecules is sufficient to overcome the intermolecular forces entirely, allowing them to escape into the gaseous phase as steam. This transition is an endothermic process, meaning it absorbs heat.
Factors Affecting the Boiling Point of Water:
Similar to the freezing point, the boiling point of water is not always fixed at 100°C. Several factors can modify this temperature:
-
Pressure: This is the most significant factor. Decreasing pressure lowers the boiling point, while increasing pressure raises it. This is why water boils at a lower temperature at high altitudes where atmospheric pressure is lower. Conversely, a pressure cooker utilizes increased pressure to raise the boiling point, allowing for faster cooking.
-
Impurities: Dissolved substances, like salts, can slightly elevate the boiling point of water. However, this effect is generally less pronounced than the effect on the freezing point.
-
Altitude: As mentioned previously, the lower atmospheric pressure at higher altitudes results in a lower boiling point. This necessitates adjustments to cooking times at high elevations.
The Significance of Water's Boiling and Freezing Points
The precise boiling and freezing points of water are fundamental to various aspects of life and scientific endeavors:
-
Climate Regulation: Water's high heat capacity, combined with its relatively high boiling and freezing points, plays a critical role in regulating Earth's climate. Large bodies of water act as thermal buffers, moderating temperature fluctuations.
-
Biology: The unique properties of water, including its high boiling and freezing points, are essential for life. Water's liquid state at Earth's typical temperatures allows for biological processes to occur. The relatively high boiling point enables water to act as a solvent, transporting nutrients and removing waste products.
-
Industry: Water's properties are exploited in numerous industrial applications, including cooling systems, steam generation for power plants, and chemical processes.
-
Cooking and Food Preparation: Understanding the boiling point of water is crucial in cooking, ensuring food is cooked properly and safely.
Understanding the Phase Diagram of Water
A phase diagram is a graphical representation showing the phases of a substance as a function of temperature and pressure. The phase diagram of water illustrates the transitions between solid, liquid, and gas states at different combinations of temperature and pressure. It's a valuable tool for visualizing how changes in these parameters affect the phase of water.
Advanced Concepts: Triple Point and Critical Point
-
Triple Point: The triple point is the unique temperature and pressure at which all three phases of water (solid, liquid, and gas) coexist in thermodynamic equilibrium. For water, this occurs at approximately 0.01°C and 611.657 Pa.
-
Critical Point: The critical point represents the temperature and pressure beyond which the distinction between liquid and gas phases disappears. For water, this occurs at approximately 374°C and 22.064 MPa. Beyond this point, water exists as a supercritical fluid, with properties intermediate between those of a liquid and a gas.
Conclusion: The Importance of Precision
The precise boiling and freezing points of water, while seemingly simple numbers, are pivotal for understanding the world around us. From the vastness of our oceans to the intricacies of cellular biology, the unique properties of water, governed by its boiling and freezing points, are foundational to life and numerous scientific and technological applications. This detailed exploration highlights not only the values themselves but the complexities and factors that influence these critical temperature thresholds, demonstrating the essential role of accurate measurements and scientific understanding in various fields. Continued research into the behavior of water at different pressures and temperatures promises to unlock even more insights into this remarkable substance.
Latest Posts
Latest Posts
-
Are Planar And Angular Nodes The Same
Mar 29, 2025
-
Which Of The Following Is Most Accurate
Mar 29, 2025
-
Name The 3 Parts Of A Dna Nucleotide
Mar 29, 2025
-
What Would Happen If The Earths Axis Was Not Tilted
Mar 29, 2025
-
What Is The Most Important Erosional Agent In Deserts
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Boiling Point And Freezing Point Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
