Is Conducting Electricity A Chemical Property
Juapaving
Mar 18, 2025 · 5 min read
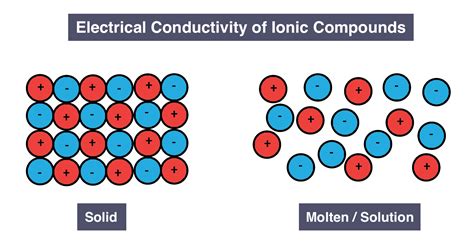
Table of Contents
Is Conducting Electricity a Chemical Property?
The question of whether electrical conductivity is a chemical property is a nuanced one, often sparking debate among students and enthusiasts of chemistry. While the answer isn't a simple "yes" or "no," understanding the intricacies of chemical properties and how they relate to electrical conductivity provides valuable insight into the behavior of matter. This comprehensive guide delves into the fascinating relationship between chemical properties, electrical conductivity, and the underlying mechanisms that govern them.
Understanding Chemical Properties
Before diving into the specifics of electrical conductivity, let's establish a clear definition of chemical properties. Chemical properties describe a substance's ability to undergo a chemical change, forming new substances with different compositions and properties. These changes often involve the breaking and forming of chemical bonds. Examples of chemical properties include flammability (ability to burn), reactivity with acids, and oxidation states. Crucially, observing a chemical property necessitates altering the substance's chemical composition.
Conductivity: A Physical or Chemical Property?
Electrical conductivity, on the other hand, is typically classified as a physical property. Physical properties describe characteristics that can be observed or measured without changing the substance's chemical composition. These include properties like density, melting point, boiling point, and, importantly, electrical conductivity. A material's ability to conduct electricity depends on its structure and the availability of mobile charge carriers (electrons or ions). This does not involve a change in the chemical composition of the material itself.
The Role of Chemical Structure in Conductivity
However, the seemingly clear distinction between physical and chemical properties blurs when considering the influence of chemical properties on electrical conductivity. The chemical composition and bonding within a material are intrinsically linked to its conductivity. Let's explore how:
1. Metallic Bonding and Conductivity
Metals are excellent conductors of electricity because of their unique metallic bonding. In metals, valence electrons are delocalized, forming a "sea" of electrons that are free to move throughout the metal lattice. This mobility of electrons allows for the easy flow of electric current. The metallic bonding itself is a chemical property, arising from the specific electronic structure and interactions of metal atoms. While the act of conducting electricity doesn't alter the chemical composition, the ability to conduct stems directly from the chemical nature of the metallic bond.
2. Ionic Compounds and Conductivity
Ionic compounds, composed of positively and negatively charged ions, exhibit conductivity only when dissolved in a solution or molten. In their solid state, the ions are held rigidly in a crystal lattice, restricting their movement. However, when dissolved or melted, the ions become mobile, allowing for the conduction of electricity. The solubility and melting point are chemical properties that govern the ability of the ionic compound to conduct electricity. Therefore, while conductivity is a physical phenomenon, the potential for conductivity is strongly influenced by chemical properties.
3. Covalent Compounds and Conductivity
Covalent compounds, characterized by the sharing of electrons between atoms, generally exhibit poor electrical conductivity. This is because the electrons are localized within covalent bonds, not free to move through the material. However, certain covalent compounds, like graphite (an allotrope of carbon), are exceptions. Graphite's layered structure allows for the delocalization of electrons within the layers, resulting in relatively good conductivity along those layers. The specific arrangement of atoms in graphite, a chemical property, is what facilitates this conductivity.
The Influence of Impurities and Doping
Introducing impurities into a material can significantly alter its electrical conductivity. This process, known as doping, is crucial in semiconductor technology. For instance, doping silicon (a semiconductor) with small amounts of phosphorus (a dopant) increases its conductivity. The chemical nature of the dopant and its interaction with the silicon lattice directly affects the material's electrical behavior. This highlights the intertwined relationship between chemical composition and electrical conductivity.
Chemical Reactions and Changes in Conductivity
While the process of conducting electricity itself doesn't inherently cause a chemical change, chemical reactions can significantly alter a substance's conductivity. For example, the corrosion of a metal involves a chemical reaction that can disrupt the metallic lattice and reduce its conductivity. Electrolysis, a process where an electric current drives a chemical reaction, demonstrates the interplay between electrical conductivity and chemical transformations. The passage of current facilitates the chemical reaction, leading to changes in the chemical composition and, in turn, conductivity.
Conductivity and the Environment
External factors like temperature and pressure can also influence conductivity. Temperature affects the kinetic energy of charge carriers. At higher temperatures, increased vibrational energy can hinder the movement of electrons or ions, leading to decreased conductivity in metals. However, in some materials like semiconductors, increased temperature can increase conductivity due to the excitation of electrons into the conduction band. The response of conductivity to temperature changes is again deeply connected to the material's chemical structure and bonding.
Conclusion: A Complex Interplay
In conclusion, while electrical conductivity is classified as a physical property, its value is deeply intertwined with a material's chemical properties. The chemical composition, bonding type, structure, and the presence of impurities all significantly impact a substance's ability to conduct electricity. The act of conducting electricity may not cause a chemical change, but the underlying chemical attributes fundamentally determine the extent and nature of that conductivity. Therefore, a complete understanding of electrical conductivity requires a holistic view that encompasses both the physical and chemical dimensions of matter. The relationship is not simply additive; it's a complex interplay where chemical properties lay the foundation for the observable physical property of electrical conductivity.
Further Exploration: Advanced Concepts
This discussion has focused on the basics. For a more in-depth understanding, exploring advanced concepts such as:
- Band theory of solids: This quantum mechanical model explains the electronic structure of solids and provides a more rigorous explanation of conductivity in different materials.
- Superconductivity: The phenomenon of zero electrical resistance at very low temperatures.
- Ionic conductivity in solids: The complexities of ion transport in solid-state electrolytes.
- Electrochemical properties: The relationship between chemical reactions and electrical potential.
These topics further solidify the intrinsic link between chemical composition and the physical phenomenon of electrical conductivity. Understanding these nuances provides a more comprehensive perspective on the behavior of materials and their interactions with electricity. This knowledge is essential across various scientific and engineering disciplines, driving innovations in areas like materials science, electronics, and energy storage.
Latest Posts
Latest Posts
-
Glucose Is Stored In Plants In The Form Of
Mar 18, 2025
-
How Many Electrons Does Oxygen Have In Its Outer Shell
Mar 18, 2025
-
Which Is Bigger Pounds Or Kilograms
Mar 18, 2025
-
What Is The Lcm Of 24 And 12
Mar 18, 2025
-
Neutral Atoms Contain Equal Numbers Of
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Conducting Electricity A Chemical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
