Neutral Atoms Contain Equal Numbers Of
Juapaving
Mar 18, 2025 · 6 min read
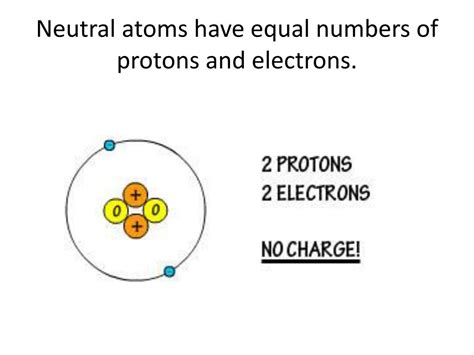
Table of Contents
Neutral Atoms Contain Equal Numbers of Protons and Electrons: A Deep Dive into Atomic Structure
Neutral atoms, the fundamental building blocks of matter, possess a fascinating characteristic: they contain an equal number of protons and electrons. This seemingly simple fact underpins much of chemistry and physics, influencing the behavior of elements and their interactions. Understanding this principle is key to unlocking a deeper appreciation of the atomic world. This article delves into the intricacies of atomic structure, exploring why neutral atoms maintain this crucial balance and the implications of this balance for the properties of matter.
The Core Components of an Atom: Protons, Neutrons, and Electrons
Before diving into the equality of protons and electrons in neutral atoms, let's review the fundamental particles that constitute an atom:
Protons: The Positive Charge Carriers
Protons reside within the atom's nucleus, a dense central region. They carry a positive electrical charge, +1, and contribute significantly to an atom's mass. The number of protons in an atom's nucleus defines its atomic number and determines the element it represents. For instance, an atom with one proton is hydrogen, an atom with two protons is helium, and so on. The atomic number is unique to each element and is a fundamental property.
Neutrons: The Neutral Partners
Neutrons, also located in the nucleus, have no electrical charge (hence the name "neutral"). They contribute to the atom's mass, but unlike protons, they do not directly influence its chemical properties. The number of neutrons in an atom can vary, leading to isotopes of the same element. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This variation in neutron number affects the atom's mass and stability, but not its fundamental chemical behavior.
Electrons: The Negative Charge Carriers
Electrons occupy the space surrounding the nucleus in regions called orbitals or electron shells. They are significantly lighter than protons and neutrons and carry a negative electrical charge, -1. The number of electrons in a neutral atom is exactly equal to the number of protons. This balance of positive and negative charges results in a net charge of zero, hence the term "neutral atom."
The Significance of Equal Protons and Electrons in Neutral Atoms
The equality of protons and electrons is crucial for several reasons:
Electrical Neutrality: The Foundation of Stability
The most fundamental consequence is the electrical neutrality of the atom. The positive charges of the protons are precisely balanced by the negative charges of the electrons. This balance ensures the atom is electrically stable, meaning it does not possess a net positive or negative charge, and it doesn't spontaneously attract or repel other charged particles with excessive force. This stability is fundamental to the formation of molecules and chemical bonds. Without this balance, atoms would be highly reactive and unstable, leading to a drastically different universe.
Chemical Behavior: Determined by Electron Configuration
While protons define the element, it's the electrons that primarily dictate the atom's chemical behavior. Electrons occupy specific energy levels or shells around the nucleus, and the arrangement of these electrons (electron configuration) determines how the atom will interact with other atoms. This interaction involves the sharing or transfer of electrons, leading to the formation of chemical bonds and the creation of molecules. Since the number of electrons equals the number of protons in a neutral atom, the electron configuration is directly linked to the atomic number, providing a predictable framework for understanding chemical reactivity.
Atomic Stability and Isotopes: The Role of Neutrons
While the number of protons and electrons determines neutrality and chemical properties, the number of neutrons plays a crucial role in nuclear stability. Isotopes, with varying neutron numbers, exhibit different stabilities. Some isotopes are stable, meaning their nuclei do not spontaneously decay. Others are radioactive, meaning their nuclei are unstable and undergo radioactive decay, emitting particles and energy to achieve a more stable configuration. This radioactive decay changes the number of protons or neutrons (and thus affecting the number of electrons in a charged atom), transforming the atom into a different element or isotope.
Ions: When the Balance is Disturbed
While neutral atoms maintain an equal number of protons and electrons, it's important to note that atoms can gain or lose electrons, leading to the formation of ions.
Cations: Positively Charged Ions
When an atom loses one or more electrons, it becomes a positively charged ion, called a cation. For example, a sodium atom (Na) can lose one electron to become a sodium cation (Na+), which now has more protons than electrons. The loss of electrons often occurs to achieve a more stable electron configuration, mimicking the electron configuration of a noble gas.
Anions: Negatively Charged Ions
Conversely, when an atom gains one or more electrons, it becomes a negatively charged ion, called an anion. For example, a chlorine atom (Cl) can gain one electron to become a chloride anion (Cl-), which now has more electrons than protons. Like cations, the gain of electrons often leads to a more stable electron configuration.
Ionic Bonds: Driven by Electrostatic Attraction
The formation of ions significantly impacts how atoms interact. Oppositely charged ions attract each other through electrostatic forces, forming ionic bonds. These bonds are crucial in the formation of many ionic compounds, such as sodium chloride (NaCl), common table salt.
The Implications of Atomic Structure and Neutral Atoms
The understanding of neutral atoms and their constituent particles has had a profound impact across various scientific fields:
Chemistry: Understanding Chemical Reactions and Bonding
In chemistry, the concept of equal protons and electrons in neutral atoms is fundamental to understanding chemical reactions and bonding. The arrangement of electrons determines an atom's reactivity, and the interactions between atoms through electron sharing or transfer lead to the formation of molecules and compounds.
Physics: Exploring Nuclear Physics and Particle Physics
In physics, the study of atomic structure is crucial in nuclear physics and particle physics. Understanding the composition of the nucleus, including the number of protons and neutrons, is essential for studying nuclear reactions, radioactive decay, and the properties of different isotopes.
Materials Science: Designing New Materials with Specific Properties
In materials science, the knowledge of atomic structure is crucial for designing new materials with specific properties. By manipulating the arrangement of atoms and their interactions, scientists can create materials with enhanced strength, conductivity, or other desired characteristics.
Medicine: Using Radioactive Isotopes for Diagnostics and Treatment
In medicine, radioactive isotopes are used in diagnostic imaging techniques like PET scans and in cancer treatments like radiotherapy. Understanding the decay properties of these isotopes is essential for ensuring their safe and effective use.
Conclusion: The Foundation of Matter
The principle that neutral atoms contain an equal number of protons and electrons is a cornerstone of our understanding of matter. This fundamental balance ensures the stability of atoms, dictates their chemical behavior, and underlies much of chemistry, physics, and materials science. Appreciating this seemingly simple fact reveals the intricate and beautiful complexity of the atomic world and its profound impact on the universe we inhabit. Further research continues to expand our understanding of the atomic realm, leading to advancements in various scientific and technological fields. The exploration of atomic structure remains a vibrant area of study, with ongoing discoveries promising to shape our future.
Latest Posts
Latest Posts
-
Square Root Of 50 Rational Or Irrational
Mar 18, 2025
-
Gas In A Liquid Solution Example
Mar 18, 2025
-
How Are Amplitude And Frequency Related
Mar 18, 2025
-
Is 25 A Multiple Of 5
Mar 18, 2025
-
How Many Valence Electrons In He
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Neutral Atoms Contain Equal Numbers Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
