How Many Electrons Does Oxygen Have In Its Outer Shell
Juapaving
Mar 18, 2025 · 5 min read
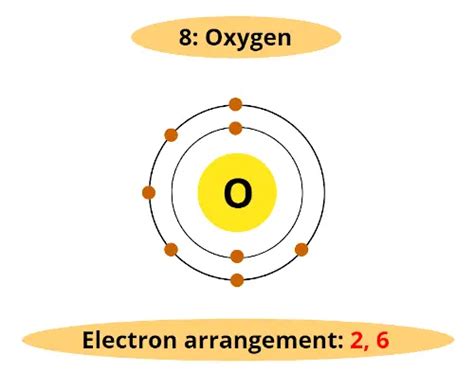
Table of Contents
How Many Electrons Does Oxygen Have in Its Outer Shell? Understanding Oxygen's Reactivity
Oxygen, a life-sustaining element, plays a crucial role in various biological and chemical processes. Understanding its electronic structure, particularly the number of electrons in its outermost shell, is key to comprehending its remarkable reactivity and its influence on the world around us. This article will delve deep into the electronic configuration of oxygen, explaining how its outer shell electrons determine its chemical behavior and its importance in various applications.
Oxygen's Atomic Structure: A Foundation for Understanding
To determine the number of electrons in oxygen's outer shell, we need to understand its atomic structure. Oxygen's atomic number is 8, meaning it has 8 protons in its nucleus and, in its neutral state, 8 electrons orbiting the nucleus. These electrons are arranged in specific energy levels or shells, following the principles of electron configuration.
Electron Shells and Subshells
Electrons occupy different energy levels, with those closest to the nucleus having the lowest energy. These levels are often referred to as shells or energy levels. The first shell (n=1) can hold a maximum of two electrons, while the second shell (n=2) can hold up to eight electrons. Within these shells are subshells, designated as s, p, d, and f, each with its own specific shape and capacity for electrons.
Oxygen's Electron Configuration
Oxygen's electron configuration is written as 1s²2s²2p⁴. Let's break this down:
- 1s²: Two electrons occupy the first shell's s subshell. The '1' represents the principal energy level (shell), 's' represents the subshell type, and '²' indicates two electrons in that subshell.
- 2s²: Two electrons occupy the second shell's s subshell.
- 2p⁴: Four electrons occupy the second shell's p subshell. The p subshell can hold a maximum of six electrons.
The Significance of the Outer Shell: Valence Electrons
The outermost shell of an atom is known as the valence shell, and the electrons in this shell are called valence electrons. These electrons are the most loosely bound to the atom and are therefore the ones involved in chemical bonding. They determine the atom's reactivity and the types of chemical bonds it can form.
Oxygen's Valence Electrons: The Key to Reactivity
Oxygen has six valence electrons. Two electrons are in the 2s subshell, and four are in the 2p subshell. This is crucial because atoms strive for a stable electron configuration, often resembling that of a noble gas with a full outer shell (eight electrons, or the duet rule for helium). Oxygen, with its six valence electrons, needs to gain two more electrons to achieve this stable octet configuration.
How Oxygen's Six Valence Electrons Influence its Chemistry
This inherent desire to gain two electrons is the driving force behind oxygen's high reactivity. It readily forms chemical bonds with other atoms to achieve a stable octet. This explains its numerous chemical interactions:
1. Formation of Ionic Bonds
Oxygen can gain two electrons from an electropositive atom (one that readily loses electrons), forming an oxide ion (O²⁻). This is an ionic bond, where there is a transfer of electrons from one atom to another. For example, in the formation of magnesium oxide (MgO), magnesium (Mg) loses two electrons to oxygen, resulting in Mg²⁺ and O²⁻ ions, held together by electrostatic attraction.
2. Formation of Covalent Bonds
Alternatively, oxygen can share electrons with other atoms to achieve an octet, forming covalent bonds. This is common in the formation of molecules like water (H₂O) and carbon dioxide (CO₂). In water, oxygen shares two electrons with each hydrogen atom, resulting in two covalent bonds. Each hydrogen atom shares one electron with oxygen to complete its duet, achieving a stable configuration.
3. Oxidation and Reduction Reactions
Oxygen's strong tendency to gain electrons makes it a powerful oxidizing agent. It readily accepts electrons from other substances, causing them to be oxidized (lose electrons). Oxygen's role as an oxidant is central to many important processes, including combustion and respiration.
Oxygen's Importance in Various Applications
Oxygen's reactivity and its ability to form various chemical bonds make it essential in a multitude of applications:
1. Respiration and Metabolism
Oxygen is crucial for aerobic respiration, the process by which living organisms convert nutrients into energy. This process involves the reduction of oxygen to water, a vital step in energy production.
2. Combustion
Oxygen is a key component in combustion reactions, where substances react with oxygen to release energy in the form of heat and light. This is utilized in power generation, industrial processes, and everyday applications like cooking and heating.
3. Medical Applications
Oxygen therapy is used to treat various respiratory conditions, providing supplementary oxygen to individuals whose bodies cannot adequately absorb oxygen from the air.
4. Industrial Processes
Oxygen is used extensively in various industrial processes, including steelmaking, welding, and the production of chemicals. Its high reactivity allows it to participate in numerous chemical reactions necessary for these processes.
5. Environmental Significance
Oxygen plays a critical role in maintaining the balance of the Earth's atmosphere and in the functioning of ecosystems. Photosynthesis, the process by which plants produce oxygen, is essential for life on Earth. The ozone layer in the stratosphere, composed of ozone (O₃), protects life from harmful ultraviolet radiation.
Conclusion: Oxygen's Six Valence Electrons - A Foundation of Life and Chemistry
Oxygen's six valence electrons are the key to understanding its remarkable reactivity and its vital role in various processes. This article has explored the fundamental principles of atomic structure, electron configuration, and chemical bonding to illustrate how oxygen's electronic structure dictates its chemical behavior. From respiration to combustion, from industrial processes to environmental significance, the influence of oxygen's six valence electrons is pervasive and undeniably crucial for life as we know it. Its role extends beyond the simple fact that it has six electrons in its outer shell; it represents a fundamental principle of chemistry and a testament to the intricate interconnectedness of matter and life. Further research into oxygen's behavior continues to reveal its complexities and importance, highlighting the fundamental significance of understanding the basic building blocks of the universe.
Latest Posts
Latest Posts
-
How Many Electrons In A Double Bond
Mar 18, 2025
-
What Is Prime Factorization Of 180
Mar 18, 2025
-
How Much Matter An Object Has
Mar 18, 2025
-
Reaction Of Iron And Hydrochloric Acid
Mar 18, 2025
-
63 Kilos Is How Many Pounds
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Oxygen Have In Its Outer Shell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
