Is Ch3 An Electron Withdrawing Group
Juapaving
Mar 29, 2025 · 5 min read
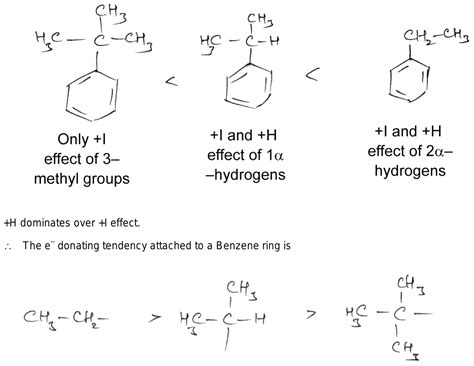
Table of Contents
Is CH3 an Electron Withdrawing Group? Understanding Inductive Effects and Hyperconjugation
The question of whether a methyl group (CH3) is electron-withdrawing or electron-donating is a nuanced one, often causing confusion among students of organic chemistry. While it's commonly understood that CH3 is electron-donating, the full picture requires a deeper dive into the concepts of inductive effects and hyperconjugation. This article will explore these concepts in detail, explaining why the seemingly simple methyl group exhibits complex electronic behavior.
Understanding Inductive Effects
Inductive effects refer to the polarization of a sigma bond caused by the difference in electronegativity between the atoms involved. Electronegativity is the ability of an atom to attract shared electrons in a covalent bond towards itself. More electronegative atoms pull electron density towards themselves, creating a partial negative charge (δ-), while the less electronegative atom develops a partial positive charge (δ+).
In the case of a carbon-carbon bond, the electronegativity difference is minimal. However, when comparing carbon to more electronegative atoms like oxygen or chlorine, the inductive effect becomes significant. These electronegative atoms withdraw electron density through the sigma bonds, making them electron-withdrawing groups.
The Role of Hyperconjugation
While inductive effects provide a basic understanding of electronic influences, hyperconjugation plays a crucial role in understanding the behavior of alkyl groups like CH3. Hyperconjugation is a stabilizing interaction involving the delocalization of electrons from a sigma bond (typically a C-H bond) into an adjacent empty or partially filled p orbital or π orbital.
In the case of a methyl group attached to a carbon atom with a partially filled p orbital (like a carbocation or a carbon atom in a double or triple bond), the C-H sigma bonds of the methyl group can donate electron density to the adjacent p orbital. This donation occurs through overlap between the sigma bonding orbital and the empty p orbital. This phenomenon is hyperconjugation, and it effectively makes the methyl group act as an electron-donating group.
Comparing Inductive and Hyperconjugative Effects in CH3
The inductive effect of a methyl group is relatively weak. Carbon is slightly more electronegative than hydrogen, leading to a very small inductive withdrawal of electron density. However, this inductive effect is largely overshadowed by the stronger hyperconjugative effect.
The hyperconjugative donation of electron density from the C-H bonds of the methyl group to the adjacent unsaturated system significantly outweighs the weak inductive electron withdrawal. This means that in most situations, the overall effect of a CH3 group is electron-donating.
CH3 as an Electron-Donating Group: Evidence and Examples
Numerous experimental observations and theoretical calculations support the electron-donating nature of the methyl group.
1. Stability of Carbocations: The stability of carbocations increases with the number of alkyl groups attached to the positively charged carbon. This is because the alkyl groups, including methyl groups, stabilize the carbocation through hyperconjugation. The more alkyl groups, the more hyperconjugative interactions, and thus the more stable the carbocation.
2. Reactivity of Aromatic Compounds: The reactivity of aromatic compounds is influenced by the substituents attached to the ring. Methyl groups are considered activating groups, meaning they increase the reactivity of the aromatic ring towards electrophilic aromatic substitution. This increased reactivity is due to the electron-donating nature of the methyl group, which increases the electron density of the aromatic ring, making it more susceptible to electrophilic attack.
3. Acid-Base Properties: Methyl groups can influence the acidity and basicity of molecules. For example, attaching methyl groups to an acid weakens its acidity, due to the electron-donating effect of the methyl groups which reduces the positive charge on the acid's conjugate base.
4. Spectroscopic Data: NMR (Nuclear Magnetic Resonance) spectroscopy can provide indirect evidence of electronic effects. The chemical shifts of protons in molecules are influenced by the electron density around them. In many cases, the chemical shifts of protons near methyl groups suggest increased electron density, supporting the electron-donating nature of the CH3 group.
Situations Where Inductive Effects Might Seem Dominant
While hyperconjugation typically dominates, there are specific situations where the weak inductive effect of a methyl group might seem more prominent. These are generally cases where hyperconjugation is less effective or absent.
-
Highly electronegative substituents: When a methyl group is attached to a highly electronegative atom, the inductive effect of the electronegative atom can sometimes outweigh the hyperconjugative effect of the methyl group. The strong electron-withdrawing ability of the electronegative atom can "trump" the donating effect of the methyl group.
-
Steric hindrance: In cases with significant steric hindrance, the ability of the methyl group to participate in hyperconjugation might be reduced. This can lead to the inductive effect having a more noticeable role.
-
Specific reaction mechanisms: The relative importance of inductive and hyperconjugative effects can also depend on the specific reaction mechanism. In some cases, the transition state of a reaction may be more sensitive to inductive effects than hyperconjugative effects.
Conclusion: The Methyl Group – A Complex Electronic Player
In summary, the methyl group (CH3) is primarily an electron-donating group due to the powerful influence of hyperconjugation. Its weak inductive electron-withdrawing effect is generally overshadowed by the electron-donating effect from hyperconjugation in most scenarios. However, understanding both inductive and hyperconjugative effects is crucial for a complete understanding of the electronic behavior of CH3 and its influence on the properties and reactivity of molecules. The relative importance of these effects can be influenced by factors like steric effects, the presence of strongly electronegative atoms, and the specifics of the chemical reaction under consideration. Therefore, while a simple answer to the question "Is CH3 an electron-withdrawing group?" might seem like "no," the reality is far more nuanced and requires a deeper examination of the interplay between inductive and hyperconjugative effects. Remembering this complexity will enhance your comprehension and application of organic chemistry principles.
Latest Posts
Latest Posts
-
Least Common Multiple 12 And 16
Apr 01, 2025
-
Ionic Compounds Are Composed Of What Particles
Apr 01, 2025
-
What Is 3 33333 As A Fraction
Apr 01, 2025
-
What Does A Prokaryotic Cell Not Have
Apr 01, 2025
-
What Are More Things About The Major Components Of Soil
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Ch3 An Electron Withdrawing Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
