Is Cellulose A Polymer Of Glucose
Juapaving
Mar 29, 2025 · 6 min read
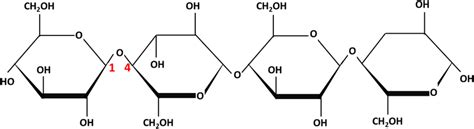
Table of Contents
Is Cellulose a Polymer of Glucose? A Deep Dive into the Structure and Properties of Cellulose
Yes, cellulose is indeed a polymer of glucose. This seemingly simple statement belies a fascinating world of chemistry, biology, and material science. Understanding the relationship between cellulose and glucose is crucial to appreciating its importance in various fields, from the textile industry to biofuel production. This article will explore this relationship in detail, examining the structure of cellulose, its properties stemming from its glucose polymer composition, and its significant roles in the natural world and human applications.
The Building Blocks: Glucose and its Isomers
Before delving into the intricacies of cellulose, it's essential to understand its fundamental building block: glucose. Glucose is a simple sugar, a monosaccharide, with the chemical formula C₆H₁₂O₆. It exists in two primary forms: α-glucose and β-glucose, which differ only in the orientation of the hydroxyl group (-OH) on carbon atom number 1. This seemingly subtle difference has profound implications for the resulting polymers.
α-glucose is the building block for starch and glycogen, both energy storage molecules in plants and animals, respectively. The α-linkages in these polymers create a helical structure, easily accessible for enzymatic breakdown and energy release.
β-glucose, on the other hand, forms the basis of cellulose. The β-linkage between glucose units results in a distinctly different structure and properties, making cellulose a vastly different molecule compared to starch and glycogen, despite sharing the same monomer.
Cellulose: A Linear Polymer of β-Glucose
Cellulose is a linear polysaccharide, meaning it's a long chain of monosaccharide units (glucose) linked together. The key difference lies in the type of linkage: β-(1→4) glycosidic bonds. These bonds connect the carbon atom at position 1 of one β-glucose molecule to the carbon atom at position 4 of the next.
This β-(1→4) linkage is crucial. It forces the glucose units into a linear, extended conformation. Many β-glucose units combine via these bonds to form long, unbranched chains, sometimes thousands of glucose units long. These chains are further organized into higher-order structures, significantly impacting cellulose's properties.
The Structure of Cellulose: From Chains to Fibrils
The individual cellulose chains aren't just randomly arranged; they are highly organized. Hydrogen bonds between the hydroxyl groups (-OH) of adjacent chains contribute significantly to cellulose's structural integrity. These hydrogen bonds create strong intermolecular forces, holding the chains together in parallel bundles called microfibrils.
These microfibrils are further aggregated into larger macrofibrils, which form the structural components of plant cell walls. The precise arrangement and orientation of these microfibrils vary depending on the plant species and the specific cell type, influencing the overall properties of the cellulose material. This hierarchical structure, from individual glucose units to macroscopic fibrils, provides cellulose with remarkable strength and resilience.
Properties of Cellulose Arising from its Glucose Polymer Structure
The unique properties of cellulose are directly derived from its glucose polymer structure and the ensuing molecular organization. These properties make cellulose a vital material in both natural and industrial contexts.
-
Strength and Rigidity: The linear structure and extensive hydrogen bonding between cellulose chains result in exceptional tensile strength and rigidity. This makes cellulose an ideal structural component in plants, providing support and protection. This strength is also exploited in various industrial applications, such as textiles and paper production.
-
Insolubility in Water: Unlike starch, cellulose is insoluble in water. This is primarily due to the extensive hydrogen bonding between cellulose chains, which prevents the water molecules from disrupting the structure and dissolving the polymer. This insolubility is essential for cellulose's role as a structural material in plants, preventing cell wall degradation in aqueous environments.
-
Crystalline Structure: The highly ordered arrangement of cellulose chains within microfibrils results in a crystalline structure. This contributes to cellulose's strength and resistance to degradation. However, the crystalline regions are interspersed with amorphous regions, influencing the accessibility of cellulose to enzymatic degradation and chemical modification.
-
Biodegradability: Despite its strength and insolubility, cellulose is ultimately biodegradable. Microorganisms produce enzymes, such as cellulases, that can break down the β-(1→4) glycosidic bonds, releasing glucose units. This biodegradability makes cellulose an environmentally friendly material, and its breakdown forms the basis of natural carbon cycling.
-
Chemical Reactivity: Cellulose contains numerous hydroxyl groups that can participate in chemical reactions. This reactivity allows for the modification of cellulose to produce a wide range of derivatives, such as cellulose acetate (used in textiles and plastics) and cellulose nitrate (used in explosives).
The Role of Cellulose in Nature and Industry
Cellulose is the most abundant organic polymer on Earth, forming the primary structural component of plant cell walls. Its prevalence reflects its critical roles in the biosphere:
-
Plant Structure and Support: Cellulose provides the structural framework for plants, giving them their rigidity and shape. Without cellulose, plants would lack the necessary support to grow upright and withstand environmental stresses.
-
Carbon Cycle: As a major component of plant biomass, cellulose plays a crucial role in the global carbon cycle. Photosynthesis fixes atmospheric carbon dioxide into glucose, which is then incorporated into cellulose. The degradation of cellulose by microorganisms returns carbon dioxide to the atmosphere, completing the cycle.
-
Dietary Fiber: Cellulose is a significant component of dietary fiber in many foods. While humans cannot digest cellulose, it plays a vital role in gut health, promoting regular bowel movements and contributing to overall digestive health.
In industry, cellulose's properties are extensively exploited:
-
Textile Industry: Cellulose is the main component of cotton, linen, and other natural fibers. These fibers are widely used in clothing production, benefiting from cellulose's strength, softness, and breathability.
-
Paper Production: Wood pulp, a major source of cellulose, is the raw material for paper manufacturing. The process involves separating and refining cellulose fibers, creating a sheet of interconnected fibers that form the paper structure.
-
Biofuel Production: Cellulose is a promising feedstock for biofuel production. Cellulose can be broken down into glucose, which can then be fermented to produce ethanol or other biofuels. Research is ongoing to develop more efficient and cost-effective methods for converting cellulose into biofuels.
-
Other Applications: Cellulose derivatives find applications in a wide range of products, including plastics, films, coatings, and adhesives. Its versatility makes it a valuable resource in various industrial sectors.
Future Directions and Research
Research on cellulose continues to expand, driven by its potential for sustainable materials and energy production. Areas of ongoing research include:
-
Improved Cellulose Degradation: Developing more efficient and cost-effective methods for breaking down cellulose is crucial for its use in biofuel production. Research focuses on optimizing enzymes and creating innovative pretreatment methods to enhance cellulose accessibility.
-
Cellulose Nanomaterials: Extracting and manipulating cellulose at the nanoscale creates materials with unique properties, such as high strength and biocompatibility. These nanomaterials are being investigated for applications in biomedical devices, composites, and advanced materials.
-
Sustainable Cellulose Production: Research is also focusing on sustainable methods for producing cellulose, including optimizing plant growth and developing innovative extraction techniques to minimize environmental impact.
Conclusion
The answer to the question "Is cellulose a polymer of glucose?" is a resounding yes. However, this simple answer only scratches the surface of a complex and fascinating molecule. The specific arrangement of β-glucose units via β-(1→4) linkages creates a unique structure with remarkable properties. These properties, ranging from exceptional strength and rigidity to biodegradability and chemical reactivity, have made cellulose an indispensable material in the natural world and a valuable resource for human applications. As research continues to unlock the full potential of this abundant polymer, cellulose will undoubtedly play an even more significant role in shaping a sustainable future.
Latest Posts
Latest Posts
-
Which Phase Do Chromosomes First Become Visible
Apr 01, 2025
-
What Is The Least Common Multiple Of 3 And 2
Apr 01, 2025
-
Difference Between Biological Science And Biology
Apr 01, 2025
-
What Is The Least Common Multiple Of 12 And 15
Apr 01, 2025
-
Carbon Dioxide And Water Combine To Form
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Cellulose A Polymer Of Glucose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
