Carbon Dioxide And Water Combine To Form
Juapaving
Apr 01, 2025 · 6 min read
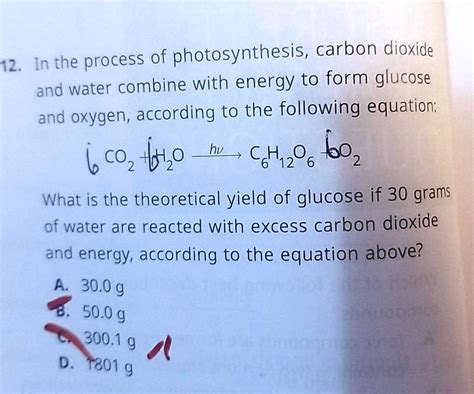
Table of Contents
Carbon Dioxide and Water Combine to Form: A Deep Dive into Carbonic Acid and its Implications
Carbon dioxide (CO₂) and water (H₂O) are two of the most abundant and essential molecules on Earth. Their interaction, seemingly simple, underpins a vast array of natural processes and has significant implications for climate change, ocean acidification, and even the biological functions of living organisms. This article will explore the chemical reaction between carbon dioxide and water, delving into the formation of carbonic acid (H₂CO₃), its properties, and its far-reaching consequences.
The Reaction: From CO₂ and H₂O to H₂CO₃
When carbon dioxide dissolves in water, a reversible chemical reaction occurs, forming carbonic acid:
CO₂(aq) + H₂O(l) ⇌ H₂CO₃(aq)
This equation shows that carbon dioxide (CO₂) in its aqueous (aq) phase reacts with liquid water (H₂O) to produce carbonic acid (H₂CO₃), also in its aqueous phase. The double arrow (⇌) indicates that this is an equilibrium reaction; it proceeds in both directions simultaneously. This means that carbonic acid can decompose back into carbon dioxide and water. The position of this equilibrium depends on several factors, including temperature, pressure, and the pH of the solution.
Understanding the Equilibrium
The equilibrium constant for this reaction is relatively small, meaning that at any given time, only a small fraction of dissolved CO₂ actually exists as H₂CO₃. Most of the dissolved CO₂ remains as CO₂ molecules, though a small portion also forms bicarbonate (HCO₃⁻) and carbonate (CO₃²⁻) ions through further reactions.
H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
HCO₃⁻(aq) ⇌ H⁺(aq) + CO₃²⁻(aq)
These subsequent reactions are crucial in determining the acidity of a solution containing dissolved CO₂. The presence of hydrogen ions (H⁺) makes the solution acidic.
The Properties of Carbonic Acid
While carbonic acid itself is a relatively weak acid, its formation and subsequent dissociation into bicarbonate and carbonate ions have profound effects on various systems. Here's a closer look at its key properties:
Weak Acidity
Carbonic acid is a weak acid, meaning it only partially dissociates in water. This is reflected in its relatively low dissociation constants (Ka values). The weak acidity of carbonic acid is fundamental to understanding its role in buffering systems, such as those found in blood and the ocean.
Decomposition and Reversibility
As mentioned earlier, the formation of carbonic acid is reversible. This reversibility is essential in many biological and geological processes. For instance, the release of CO₂ from carbonic acid in plants during photosynthesis relies on this reversible reaction.
Biological Relevance
The formation and breakdown of carbonic acid play critical roles in several biological systems:
-
Carbon Dioxide Transport in Blood: Carbonic anhydrase, an enzyme present in red blood cells, significantly accelerates the conversion of CO₂ to H₂CO₃ and vice versa. This process is essential for efficient transport of CO₂ from the tissues to the lungs for exhalation.
-
Photosynthesis: Plants absorb CO₂ from the atmosphere. The dissolved CO₂ then forms carbonic acid which is utilized in the process of photosynthesis.
-
Regulation of Blood pH: The carbonic acid-bicarbonate buffer system helps maintain the pH of blood within a narrow range, crucial for the proper functioning of various biological processes.
The Implications of Carbonic Acid Formation: Ocean Acidification and Climate Change
The interaction between CO₂ and water has far-reaching environmental implications, most notably in relation to:
Ocean Acidification
The increasing atmospheric concentration of CO₂ due to human activities is leading to a significant increase in the amount of CO₂ absorbed by the oceans. This absorption results in the formation of carbonic acid, lowering the ocean's pH. This process, known as ocean acidification, has severe consequences for marine ecosystems, particularly for organisms that build shells and skeletons from calcium carbonate, such as corals, shellfish, and plankton. The increased acidity makes it more difficult for these organisms to build and maintain their shells, threatening their survival and disrupting the entire marine food web.
Understanding the impact: The increased concentration of H⁺ ions from carbonic acid reduces the availability of carbonate ions (CO₃²⁻), which are essential for calcium carbonate formation (CaCO₃). This makes it harder for marine organisms to build their shells and skeletons, resulting in slower growth, weakening of structures, and increased mortality.
Climate Change
The greenhouse effect is primarily driven by greenhouse gases, with CO₂ being a major contributor. The increased concentration of atmospheric CO₂ traps heat in the Earth's atmosphere, leading to global warming and climate change. Understanding the behavior of CO₂ in the atmosphere and its interaction with water is crucial for predicting and mitigating the effects of climate change.
The carbon cycle: The carbon cycle describes the continuous movement of carbon atoms between the atmosphere, oceans, land, and living organisms. The formation of carbonic acid is a vital part of this cycle. Understanding its dynamics is critical in developing effective climate change mitigation strategies.
Carbonic Acid in Everyday Life and Industrial Applications
Beyond its environmental impact, carbonic acid also plays a role in various aspects of our daily lives and industrial processes:
Carbonated Beverages
The fizz in carbonated drinks comes from dissolved CO₂, which forms carbonic acid in the liquid. The release of CO₂ when the bottle is opened is simply the reverse reaction of CO₂ dissolving in water.
Fire Extinguishers
Some fire extinguishers utilize carbonic acid to extinguish fires. The release of CO₂ smothers the flames by displacing oxygen.
Chemical Industry
Carbonic acid and its derivatives are used as raw materials or intermediates in various industrial processes.
Further Research and Future Directions
The ongoing research on carbonic acid and its related reactions continues to unveil new insights into its complex role in various natural and man-made systems. Future research will likely focus on:
-
Improving models of ocean acidification: Developing more accurate models to predict the future impacts of ocean acidification on marine ecosystems.
-
Developing strategies to mitigate CO₂ emissions: Developing and implementing effective strategies to reduce CO₂ emissions and lessen the effects of climate change.
-
Exploring alternative uses of carbonic acid and its derivatives: Investigating potential applications of carbonic acid and its derivatives in sustainable technologies.
-
Investigating the impact on specific ecosystems: Focusing research efforts on understanding the unique effects of ocean acidification on vulnerable ecosystems.
Conclusion
The seemingly simple reaction between carbon dioxide and water to form carbonic acid has profound implications for our planet. Understanding the properties, reactions, and effects of carbonic acid is vital to addressing crucial environmental challenges, such as ocean acidification and climate change. Further research and ongoing efforts to mitigate CO₂ emissions are crucial for safeguarding our environment and preserving the health of our planet. The intricate relationship between CO₂, H₂O, and H₂CO₃ highlights the interconnectedness of Earth's systems and the importance of a holistic approach to environmental stewardship.
Latest Posts
Latest Posts
-
120 Sq Meters To Sq Ft
Apr 02, 2025
-
The Shared Endpoint Of Two Rays Is Called The
Apr 02, 2025
-
You Can Increase The Strength Of An Electromagnet By
Apr 02, 2025
-
Metals That Can Be Cut With Knife
Apr 02, 2025
-
Magnetic Field From A Current Loop
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Carbon Dioxide And Water Combine To Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
