Is Carbonic Acid A Weak Electrolyte
Juapaving
Mar 31, 2025 · 6 min read
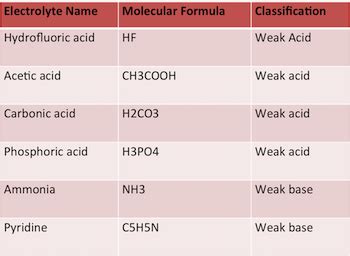
Table of Contents
Is Carbonic Acid a Weak Electrolyte? A Deep Dive into its Dissociation and Properties
Carbonic acid (H₂CO₃) is a crucial compound in various biological and geological processes. Understanding its properties, particularly its electrolytic behavior, is essential for comprehending these processes. This article delves deep into the question: Is carbonic acid a weak electrolyte? We'll explore its dissociation, equilibrium constants, and the factors influencing its behavior in solution.
Understanding Electrolytes and Their Classification
Before examining carbonic acid specifically, let's establish a foundational understanding of electrolytes. Electrolytes are substances that, when dissolved in a polar solvent like water, produce a solution that conducts electricity. This conductivity arises from the presence of freely moving ions – positively charged cations and negatively charged anions.
Electrolytes are classified based on the extent of their dissociation into ions:
-
Strong Electrolytes: These substances completely dissociate into ions in solution. Examples include strong acids (like HCl and HNO₃), strong bases (like NaOH and KOH), and most soluble salts.
-
Weak Electrolytes: These substances only partially dissociate into ions in solution. A significant portion remains undissociated as neutral molecules. Examples include weak acids (like acetic acid and carbonic acid), weak bases (like ammonia), and some sparingly soluble salts.
-
Non-Electrolytes: These substances do not dissociate into ions in solution and do not conduct electricity. Examples include sugars (like glucose and sucrose) and alcohols (like ethanol).
Carbonic Acid: A Weak Acid and a Weak Electrolyte
The answer to our central question is a resounding yes, carbonic acid is a weak electrolyte. This is because it's a weak acid, meaning it only partially dissociates in water. The dissociation reaction is as follows:
H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
The double arrow (⇌) indicates that the reaction is an equilibrium, meaning it proceeds in both directions simultaneously. At equilibrium, a significant portion of the carbonic acid remains undissociated as H₂CO₃ molecules. Only a small fraction dissociates into hydrogen ions (H⁺) and bicarbonate ions (HCO₃⁻).
This partial dissociation is quantified by the acid dissociation constant, Kₐ₁. This constant represents the ratio of the concentrations of the products (H⁺ and HCO₃⁻) to the concentration of the reactant (H₂CO₃) at equilibrium. The Kₐ₁ value for carbonic acid is relatively small, typically around 4.3 × 10⁻⁷ at 25°C. This small value confirms the weak nature of carbonic acid as an electrolyte.
Factors Affecting Carbonic Acid Dissociation
Several factors influence the extent of carbonic acid dissociation:
1. Concentration:
The concentration of carbonic acid in the solution affects the degree of dissociation. According to Le Chatelier's principle, increasing the concentration of H₂CO₃ will shift the equilibrium to the right, increasing the concentration of H⁺ and HCO₃⁻ ions, but the percentage dissociation remains the same. Conversely, decreasing the concentration will shift the equilibrium to the left, reducing the concentration of ions.
2. Temperature:
Temperature also plays a role. Increasing the temperature generally increases the dissociation of weak acids like carbonic acid. This is because the dissociation is endothermic (absorbs heat), and increasing the temperature favors the endothermic reaction.
3. Presence of Common Ions:
The presence of common ions in the solution can suppress the dissociation of carbonic acid. This is known as the common ion effect. For example, adding sodium bicarbonate (NaHCO₃) to a carbonic acid solution will increase the concentration of bicarbonate ions (HCO₃⁻), shifting the equilibrium to the left and decreasing the dissociation of carbonic acid.
4. pH of the Solution:
The pH of the solution significantly impacts the dissociation of carbonic acid. In acidic solutions (low pH), the equilibrium shifts to the left, reducing dissociation. In alkaline solutions (high pH), the equilibrium shifts to the right, increasing dissociation. This relationship highlights the importance of considering the overall solution environment when studying carbonic acid’s behavior.
5. Pressure of Carbon Dioxide:
Carbonic acid's formation is intimately linked to the dissolution of carbon dioxide in water:
CO₂(g) + H₂O(l) ⇌ H₂CO₃(aq)
This equilibrium is heavily influenced by the partial pressure of CO₂. Higher CO₂ pressure shifts the equilibrium to the right, increasing the concentration of H₂CO₃, consequently increasing the concentration of H+ and HCO3- ions, though the percentage dissociation remains largely unchanged. This is particularly relevant in understanding the acidification of oceans due to increased atmospheric CO₂ levels.
The Second Dissociation of Carbonic Acid
Carbonic acid undergoes a second dissociation, albeit to a much smaller extent:
HCO₃⁻(aq) ⇌ H⁺(aq) + CO₃²⁻(aq)
This second dissociation is characterized by a much smaller acid dissociation constant, Kₐ₂, typically around 4.7 × 10⁻¹¹ at 25°C. This means that the concentration of carbonate ions (CO₃²⁻) is significantly lower than the concentration of bicarbonate ions (HCO₃⁻) in solution. This second dissociation is often less significant in many applications compared to the first dissociation.
Biological and Geological Significance of Carbonic Acid's Weak Electrolyte Nature
The weak electrolyte nature of carbonic acid is crucial in various biological and geological contexts:
-
Blood Buffer System: Carbonic acid and bicarbonate ions constitute a vital buffer system in human blood. This buffer system helps maintain the pH of blood within a narrow physiological range, crucial for proper enzyme function and overall biological processes. The weak dissociation of carbonic acid allows it to effectively absorb excess H⁺ ions, preventing significant pH changes.
-
Ocean Acidification: The absorption of atmospheric CO₂ by the oceans increases the concentration of carbonic acid, leading to ocean acidification. The weak electrolyte nature of carbonic acid plays a crucial role in this process, as the increased concentration of H⁺ ions lowers the ocean's pH, affecting marine organisms and ecosystems.
-
Carbonate Rock Formation: Carbonic acid plays a significant role in the weathering of rocks, particularly carbonate rocks like limestone and marble. The weak dissociation allows it to dissolve these rocks slowly over geological timescales.
-
Cave Formation: The dissolution of limestone by slightly acidic groundwater, containing carbonic acid, is the primary mechanism behind the formation of caves. The weak dissociation and equilibrium nature of the process leads to gradual dissolution and the intricate formations found within caves.
Conclusion: Carbonic Acid's Weak Electrolyte Behavior and its Broader Implications
In conclusion, carbonic acid is undeniably a weak electrolyte due to its partial dissociation in water and its relatively small acid dissociation constants (Kₐ₁ and Kₐ₂). This weak electrolyte behavior is far from trivial. It significantly influences its role in various biological and geological processes, from maintaining blood pH to driving the formation of caves and impacting ocean acidification. A deeper understanding of carbonic acid's dissociation and the factors that affect it is crucial for addressing environmental challenges and unraveling complex natural processes. Further research into the precise effects of temperature, pressure, and ionic strength on carbonic acid's dissociation remains a vital area of study for various scientific disciplines.
Latest Posts
Latest Posts
-
7 875 Rounded To The Nearest Hundredth
Apr 02, 2025
-
How Many Parents Are Needed For Asexual Reproduction
Apr 02, 2025
-
Least Common Multiple Of 35 And 49
Apr 02, 2025
-
What Is Meant By The Simplest Formula Of A Compound
Apr 02, 2025
-
What Is The Largest Gland In The Body
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Carbonic Acid A Weak Electrolyte . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
