Is Calcium A Metalloid Or Metal
Juapaving
Mar 04, 2025 · 6 min read
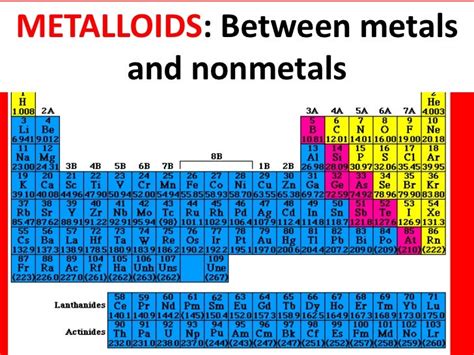
Table of Contents
Is Calcium a Metalloid or Metal? A Deep Dive into Calcium's Properties
The question of whether calcium is a metalloid or a metal is easily answered: calcium is a metal. However, understanding why it's definitively a metal, and exploring the nuances of its properties relative to metalloids, requires a deeper dive into the fascinating world of chemical classification. This article will explore the characteristics that define metals and metalloids, comparing them to calcium's properties to definitively establish its classification and dispel any confusion.
Understanding Metals and Metalloids
Before we delve into calcium's specific characteristics, let's establish a clear understanding of what distinguishes metals from metalloids. These two classes of elements share some similarities, but key differences in their physical and chemical properties lead to their distinct classifications on the periodic table.
Properties of Metals:
-
Excellent Conductors of Heat and Electricity: Metals are renowned for their ability to efficiently transfer both heat and electricity. This is due to the structure of their atoms and the free movement of electrons within their metallic bonding. This characteristic is fundamental to many applications of metals in wiring, heating elements, and various electronic components.
-
High Malleability and Ductility: Metals can be easily shaped (malleability) by hammering or rolling, and drawn into wires (ductility), without breaking. This is a direct consequence of the sea of delocalized electrons that allow metal atoms to slide past one another without disrupting the metallic bonding.
-
High Tensile Strength: Metals generally possess high tensile strength, meaning they can withstand significant pulling forces before breaking. This property is crucial in construction, engineering, and numerous industrial applications.
-
Luster: Most metals exhibit a characteristic metallic luster—a shiny appearance. This is because of the interaction of light with the delocalized electrons in the metal's structure.
-
High Density: Compared to non-metals, metals tend to have high densities, meaning they possess a large amount of mass packed into a relatively small volume.
-
Solid at Room Temperature (Except Mercury): The vast majority of metals are solid at room temperature, with mercury being the notable exception. Their strong metallic bonds contribute to their solid state.
Properties of Metalloids:
Metalloids, also known as semimetals, occupy a fascinating middle ground between metals and non-metals on the periodic table. Their properties are often intermediate, exhibiting characteristics of both metals and non-metals depending on the specific conditions.
-
Semiconductors: This is perhaps the most defining characteristic of metalloids. They are neither good conductors nor good insulators of electricity; their conductivity lies somewhere in between, and can be significantly influenced by temperature, light, or the presence of impurities. This semiconducting property is essential in the electronics industry, forming the basis of transistors and integrated circuits.
-
Variable Properties: Metalloids can exhibit properties that are sometimes metallic and sometimes non-metallic, depending on the context. For example, their appearance can vary, and their reactivity can differ significantly under different conditions.
-
Brittle: Metalloids are generally brittle and lack the malleability and ductility of metals.
Calcium's Properties: A Definitive Metal
Now let's examine the properties of calcium to firmly establish its classification as a metal.
Physical Properties of Calcium:
-
Silvery-White Appearance: Calcium is a silvery-white metal with a relatively soft texture. This lustrous appearance is a classic indicator of a metal.
-
Excellent Conductor of Heat and Electricity: Calcium exhibits excellent conductivity for both heat and electricity, a hallmark characteristic of metals. This is due to the ease with which electrons move within its metallic structure.
-
Malleable and Ductile: Although less so than some other metals, calcium possesses measurable malleability and ductility. It can be shaped and drawn into wires, albeit with more difficulty than highly malleable metals like gold or copper.
-
Low Density Compared to Transition Metals: While still denser than many non-metals, calcium has a relatively low density compared to many transition metals. This doesn't negate its classification as a metal; density varies significantly within the metal category.
-
Melting Point and Boiling Point: Calcium has a relatively low melting point (842 °C) and boiling point (1484 °C) compared to many other metals. However, these values are still considerably higher than those of non-metals.
Chemical Properties of Calcium:
-
Highly Reactive: Calcium is a highly reactive metal, readily reacting with oxygen in the air to form calcium oxide (CaO). This reactivity is characteristic of many alkaline earth metals.
-
Reaction with Water: Calcium reacts vigorously with water, producing calcium hydroxide (Ca(OH)₂) and hydrogen gas. This is another indicator of its metallic nature, as many non-metals do not readily react with water.
-
Formation of Ions: Calcium readily loses two electrons to form a +2 ion (Ca²⁺). This tendency to lose electrons, forming positive ions, is a defining characteristic of metals.
-
Oxidation State: Calcium consistently exhibits a +2 oxidation state, further supporting its classification as a metal with a predictable electron donation behavior.
Comparing Calcium to Metalloids: The Clear Distinction
The properties discussed above clearly demonstrate that calcium lacks the characteristics typically associated with metalloids. It does not exhibit semiconducting behavior; instead, it's a good conductor of heat and electricity. Its malleability, ductility, and metallic luster further solidify its classification as a metal. It doesn't display the intermediate properties that define metalloids; its behavior is unequivocally metallic.
Addressing Potential Confusion: The Periodic Table's Position
Sometimes, the proximity of an element to the metalloid region on the periodic table can lead to confusion. Calcium's position near the boundary between metals and non-metals might lead some to question its classification. However, its properties, as extensively discussed above, firmly place it within the metal category. The periodic table serves as a valuable guide but is not the sole determinant of an element's classification. Its properties must be examined comprehensively.
Conclusion: Calcium – A True Metal
In conclusion, the evidence overwhelmingly supports the classification of calcium as a metal. Its physical properties, such as its metallic luster, high conductivity, malleability, and ductility, along with its chemical properties, including its reactivity and tendency to form positive ions, firmly place it within the metal category. While its position on the periodic table might raise questions, a thorough examination of its properties leaves no doubt – calcium is definitively a metal, not a metalloid. Understanding these properties is key to appreciating its diverse applications and importance in various fields, from biological processes to industrial materials. The distinct differences between metals and metalloids, highlighted in this analysis, underscore the importance of evaluating an element's properties holistically rather than relying solely on its periodic table position for accurate classification. This comprehensive examination of calcium's properties aims to provide clarity and reinforce a fundamental concept in chemistry.
Latest Posts
Latest Posts
-
Why Cant Aluminum Be Reduced With Carbon
Mar 04, 2025
-
Which Of The Following Is Insoluble In Water
Mar 04, 2025
-
How Do Hypotheses Differ From Theories
Mar 04, 2025
-
What Is The Least Common Multiple Of 7 And 10
Mar 04, 2025
-
Electric Charges That Are Different Attract Each Other True False
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Is Calcium A Metalloid Or Metal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
