Is Ammonium Nitrate Ionic Or Covalent
Juapaving
Apr 06, 2025 · 6 min read
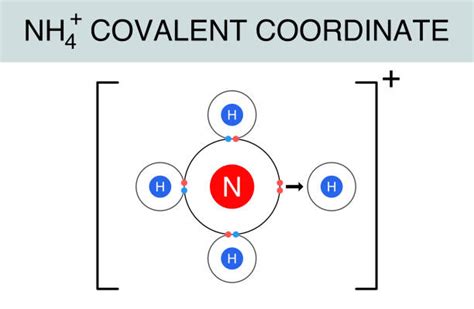
Table of Contents
Is Ammonium Nitrate Ionic or Covalent? A Deep Dive into Chemical Bonding
Ammonium nitrate (NH₄NO₃) is a fascinating chemical compound that sparks considerable debate regarding its bonding nature. Is it purely ionic, purely covalent, or a blend of both? This question delves into the fundamental concepts of chemical bonding, exploring the intricacies of ionic and covalent interactions and how they manifest in ammonium nitrate. Understanding this requires a closer look at the individual components and the forces that hold them together.
Understanding Ionic and Covalent Bonds
Before we delve into the specifics of ammonium nitrate, let's establish a clear understanding of ionic and covalent bonds.
Ionic Bonds: The Electrostatic Attraction
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. This occurs when one atom, typically a metal, readily loses electrons to achieve a stable electron configuration (a full outer shell). This atom becomes a positively charged cation. Another atom, often a non-metal, readily gains these electrons, becoming a negatively charged anion. The strong electrostatic forces between these ions create the ionic bond. Classic examples include sodium chloride (NaCl) and magnesium oxide (MgO). Ionic compounds generally have high melting and boiling points and are often soluble in water.
Covalent Bonds: Sharing is Caring
Covalent bonds, on the other hand, involve the sharing of electrons between two atoms, usually non-metals. This sharing allows both atoms to achieve a stable electron configuration. The shared electrons are attracted to the nuclei of both atoms, creating a bond. The strength of a covalent bond depends on the degree of electron sharing, with stronger bonds resulting from more equal sharing. Examples include water (H₂O) and methane (CH₄). Covalent compounds often have lower melting and boiling points compared to ionic compounds and exhibit varying solubilities in water.
Deconstructing Ammonium Nitrate: A Polyatomic Perspective
Ammonium nitrate is unique because it contains polyatomic ions. These are ions composed of two or more atoms covalently bonded together, carrying an overall charge. In ammonium nitrate, we have two:
1. Ammonium Ion (NH₄⁺): A Covalent Heart with an Ionic Charge
The ammonium ion consists of a central nitrogen atom covalently bonded to four hydrogen atoms. Nitrogen, with five valence electrons, shares one electron with each hydrogen atom, achieving a stable octet. However, the nitrogen atom also carries a positive charge because it has effectively lost one electron in this bonding arrangement. This positive charge is distributed across the entire ion, making it a cation. The bonds within the ammonium ion are predominantly covalent.
2. Nitrate Ion (NO₃⁻): Resonance and Covalent Bonding
The nitrate ion is composed of a central nitrogen atom covalently bonded to three oxygen atoms. Nitrogen shares electrons with each oxygen atom. However, the arrangement is more complex due to resonance. Resonance means that the actual structure of the nitrate ion is a hybrid of multiple contributing structures, with the electrons delocalized across the entire ion. This delocalization leads to an overall negative charge distributed across the ion, making it an anion. The bonds within the nitrate ion are also predominantly covalent.
The Ionic Interaction: Bridging the Covalent Units
The ammonium ion (NH₄⁺) and the nitrate ion (NO₃⁻) are held together by strong electrostatic forces, forming an ionic bond. This ionic interaction arises from the positive charge on the ammonium ion and the negative charge on the nitrate ion. This is the primary force holding the ammonium nitrate crystal lattice together.
So, Is Ammonium Nitrate Ionic or Covalent? The Answer is Both!
The bonding in ammonium nitrate is best described as a combination of both ionic and covalent bonding. Within each polyatomic ion (ammonium and nitrate), the bonds are primarily covalent. However, the attraction between the positively charged ammonium ion and the negatively charged nitrate ion is decidedly ionic. Therefore, ammonium nitrate exhibits characteristics of both ionic and covalent compounds.
Properties Reflecting the Dual Bonding Nature
The properties of ammonium nitrate reflect this dual bonding nature:
-
Solubility in Water: Ammonium nitrate is highly soluble in water, a characteristic typically associated with ionic compounds. The polar water molecules effectively interact with the charged ammonium and nitrate ions, leading to dissolution.
-
Melting Point: Ammonium nitrate has a relatively high melting point compared to many purely covalent compounds. This indicates the presence of strong electrostatic forces between the ions.
-
Crystal Structure: The crystal structure of ammonium nitrate is an ordered arrangement of ammonium and nitrate ions held together by ionic forces.
-
Reactivity: Ammonium nitrate is a powerful oxidizing agent due to the presence of the nitrate ion. Its reactivity is related to the covalent bonding within the nitrate ion and the ability of the nitrate ion to accept electrons, facilitating oxidation-reduction reactions. This makes it a crucial component in various applications, including fertilizers and explosives.
Understanding the Nuances: A Deeper Look at Electronegativity
The concept of electronegativity provides further insight. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. The difference in electronegativity between atoms determines the character of the bond:
- Large difference: Leads to ionic bonds.
- Small difference: Leads to covalent bonds.
- Intermediate difference: Leads to polar covalent bonds (partial ionic character).
In ammonium nitrate, the N-H bonds within the ammonium ion have a small electronegativity difference, resulting in polar covalent bonds. The N-O bonds within the nitrate ion also exhibit a small electronegativity difference, leading to polar covalent bonds with significant resonance. The interaction between the ammonium and nitrate ions involves a substantial electronegativity difference, resulting in ionic character.
Applications Highlighting the Dual Nature
The versatile properties of ammonium nitrate, arising from its dual bonding nature, lead to its widespread applications:
-
Agriculture: It is a primary ingredient in many nitrogen-based fertilizers, providing essential nitrogen for plant growth. This application exploits its solubility in water and the availability of nitrogen from the ammonium ion and the nitrate ion to plant roots.
-
Explosives: Ammonium nitrate is also used as an oxidizer in explosives and blasting agents. The strong oxidizing power of the nitrate ion, combined with the presence of a readily combustible ammonium ion, makes it a powerful explosive. However, this is carefully managed to avoid unintended consequences.
-
Other Industrial Uses: Ammonium nitrate finds applications in various industrial processes, from cold packs (endothermic dissolution in water) to production of nitrous oxide.
Conclusion: A Balanced Perspective
In conclusion, ammonium nitrate is not simply ionic or covalent; it's a compelling example of a compound with both types of bonding. The covalent bonding within the polyatomic ions forms the foundation, while the ionic interaction between these ions provides the overall structure and many of its observed properties. Understanding this dual nature is crucial for comprehending its reactivity, applications, and overall chemical behavior. This fascinating compound offers a valuable illustration of the complexities of chemical bonding and its implications in the real world. Further study into the specific interactions within the crystal lattice and its dynamics under different conditions could reveal even more about this widely used substance.
Latest Posts
Latest Posts
-
Why Is Blood Regarded As A Connective Tissue
Apr 07, 2025
-
Prime Implicants And Essential Prime Implicants
Apr 07, 2025
-
How Tall Is 49 Inches In Feet
Apr 07, 2025
-
The Ratio Between Map Distance And Ground Distance Is Called
Apr 07, 2025
-
How Do You Find The Perimeter Of A Right Triangle
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Is Ammonium Nitrate Ionic Or Covalent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.