Is Alcohol A Pure Substance Or A Mixture
Juapaving
Mar 29, 2025 · 5 min read
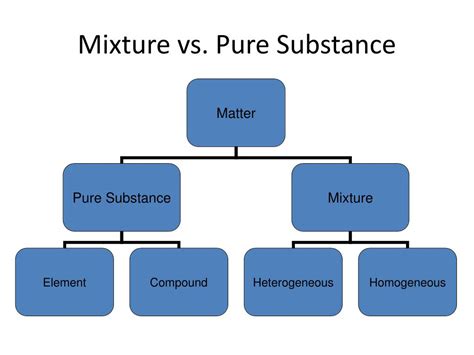
Table of Contents
Is Alcohol a Pure Substance or a Mixture? A Deep Dive into the Chemistry of Alcoholic Beverages
The question of whether alcohol is a pure substance or a mixture is deceptively complex. The simple answer depends entirely on which alcohol and how it's prepared. Let's delve into the fascinating chemistry to understand the nuances.
Defining Pure Substances and Mixtures
Before we dissect the alcoholic world, it's crucial to establish clear definitions:
-
Pure Substance: A pure substance has a fixed chemical composition throughout. It cannot be separated into simpler components by physical methods (like filtration or distillation). Examples include elements (like gold or oxygen) and compounds (like water or table salt). A pure substance has a unique set of physical and chemical properties, such as a specific melting and boiling point.
-
Mixture: A mixture is a combination of two or more substances that are physically combined but not chemically bonded. The components retain their individual properties, and the composition can vary. Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water).
The Case of Ethanol: The "Alcohol" in Alcoholic Beverages
When most people say "alcohol," they mean ethanol (CH₃CH₂OH), the type of alcohol found in alcoholic beverages. Pure ethanol is a pure substance, a single chemical compound with a specific molecular structure. It's colorless, volatile, flammable, and has a distinct odor and taste. It boils at 78.4°C and freezes at -114.1°C. These properties are consistent and unchanging.
However, the ethanol found in alcoholic beverages is rarely, if ever, pure. This is where things get interesting.
Alcoholic Beverages: Complex Mixtures
The ethanol in drinks like wine, beer, and spirits is almost always part of a complex mixture. Let's examine the components:
1. Water: A Major Component
Water (H₂O) is a significant component in most alcoholic beverages. It's not just a diluent; it plays a crucial role in taste, mouthfeel, and the overall experience. The water content varies depending on the type of beverage. For example, beer generally has a higher water content than whiskey.
2. Congeners: Adding Complexity and Flavor
Congeners are other organic compounds produced during fermentation or added during the aging process. These contribute significantly to the flavor, aroma, and color of alcoholic beverages. Congeners are a diverse group, including:
-
Alcohols: Besides ethanol, other alcohols like methanol, propanol, and butanol might be present in trace amounts. These can affect taste and even toxicity if present in high concentrations.
-
Aldehydes: These contribute to the aroma and flavor profile, often contributing fruity or spicy notes. Acetaldehyde is a common example.
-
Esters: These are often responsible for fruity and floral aromas.
-
Acids: Organic acids like acetic acid (vinegar) and lactic acid contribute to sourness and complexity.
-
Ketones: These compounds can add various flavor notes depending on their structure.
The specific types and amounts of congeners vary greatly depending on factors such as the raw materials used, fermentation methods, distillation techniques, and aging processes. This explains why a Scotch whisky tastes different from a vodka, even though both primarily contain ethanol. The congeners are what make each beverage unique.
3. Other Additives: Enhancing the Experience (or Not)
Besides the naturally occurring components, some alcoholic beverages may contain added substances, further solidifying their nature as mixtures:
-
Sugar: Added sugar is common in some liqueurs, sweet wines, and ready-to-drink cocktails, increasing sweetness and often viscosity.
-
Artificial Sweeteners: Some alcoholic beverages use artificial sweeteners to reduce sugar content.
-
Colorings: Artificial colorings might be added to enhance the visual appeal of certain drinks.
-
Flavorings: Natural or artificial flavorings can further customize the taste.
-
Preservatives: Preservatives extend the shelf life of the beverage.
The Distillation Process: Refining, But Not Purifying Completely
Distillation is a crucial step in the production of many spirits. It separates ethanol from other components based on their boiling points. While distillation increases the ethanol concentration, it doesn't yield perfectly pure ethanol. Even the purest spirits contain trace amounts of other compounds—residual congeners that contribute to their unique character. The distillation process itself can even create or modify certain congeners.
Analyzing the Mixtures: Techniques for Understanding Alcoholic Beverages
The composition of alcoholic beverages is analyzed using various techniques:
-
Gas Chromatography (GC): This separates and identifies the various volatile compounds present, including congeners.
-
High-Performance Liquid Chromatography (HPLC): This is used to analyze non-volatile compounds.
-
Mass Spectrometry (MS): Often coupled with GC or HPLC, MS provides precise identification and quantification of the compounds.
These analytical techniques highlight the complex nature of alcoholic beverages, confirming their status as mixtures rather than pure substances.
Conclusion: It Depends!
Therefore, the answer to the question "Is alcohol a pure substance or a mixture?" depends on the context. Pure ethanol is a pure substance, a single chemical compound. However, alcoholic beverages are invariably complex mixtures containing ethanol, water, and a variety of congeners, along with sometimes added substances. The specific composition determines the unique characteristics of each beverage, making the world of alcoholic drinks a fascinating study in chemistry and sensory experience. The journey from pure ethanol to the complexities of a single malt whiskey perfectly illustrates the transformative power of mixture. Understanding this distinction is critical for appreciating the subtleties of alcoholic beverages and for those interested in the scientific aspects of brewing, distilling, and winemaking. The vast array of flavors and aromas found in alcoholic drinks is a testament to the intricate interplay of various chemical compounds within these mixtures, making each sip a complex and nuanced experience.
Latest Posts
Latest Posts
-
A Rectangle Is Sometimes A Square
Mar 31, 2025
-
The Two Purines In Dna Are
Mar 31, 2025
-
A To Z In Cursive Writing
Mar 31, 2025
-
What Is The Purpose Of Mitosis In Single Celled Organisms
Mar 31, 2025
-
What Is The Difference Between A Land And Sea Breeze
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Alcohol A Pure Substance Or A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
